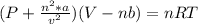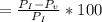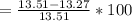Physics, 05.09.2019 16:10 skgoldsmith
According to the ideal gas law, a 1.074 mol sample of oxygen gas in a 1.746 l container at 267.6 k should exert a pressure of 13.51 atm. what is the percent difference between the pressure calculated using the van der waals' equation and the ideal pressure? for o2 gas, a = 1.360 l2atm/mol2 and b = 3.183×10-2 l/mol.

Answers: 1


Another question on Physics

Physics, 22.06.2019 13:00
Discuss how the hardness or softness of the landing surface is related to the time required to stop the egg
Answers: 1

Physics, 22.06.2019 16:30
Each neuron shown in this figure innervates a group of muscle fibers. what is the term for a group of muscle fibers innervated by a single neuron?
Answers: 1

Physics, 23.06.2019 11:00
The temperature of a solution can change the solubility of a substance. the chart shows the solubility of the same solute at different temperatures in water. based on the chart, which solution was at the optimal temperature to allow for the greatest solubility? w x y z
Answers: 2

Physics, 23.06.2019 15:20
Which feature is used to classify a rock based on its composition?
Answers: 2
You know the right answer?
According to the ideal gas law, a 1.074 mol sample of oxygen gas in a 1.746 l container at 267.6 k s...
Questions

Physics, 26.11.2021 02:30



Mathematics, 26.11.2021 02:30




Social Studies, 26.11.2021 02:30

Biology, 26.11.2021 02:30



Mathematics, 26.11.2021 02:30

Mathematics, 26.11.2021 02:30


SAT, 26.11.2021 02:30



Mathematics, 26.11.2021 02:30