
Physics, 04.09.2019 22:10 faithyholcomb
What is the enthalpy change (in kj) of a chemical reaction that raises the temperature of 250.0 ml of solution having a density of 1.25 g/ml by 3.33 °c? (the specific heat of the solution is 3.74 joules/gram-k.) what is the enthalpy change (in kj) of a chemical reaction that raises the temperature of 250.0 ml of solution having a density of 1.25 g/ml by 3.33 °c? (the specific heat of the solution is 3.74 joules/gram-k.) -3.89 -7.43 8.20 6.51 -12.51

Answers: 3


Another question on Physics

Physics, 21.06.2019 19:30
Molten iron fills a mould, which has a volume of 200 cm cubed. calculate the volume when the iron cools and solidifies. molten iron has a density of 7.0g/cm cubed. in its solid state, iron has a density of 8.0g/cm cubed.
Answers: 3

Physics, 22.06.2019 06:30
2kg of refrigerant 134a undergoes a polytropic process in a piston-cylinder assembly from an initial state of saturated vapor at 2 bar to a final state of 12 bar, 80 degree c. a)determine the work for the process in kj. b)sketch the process on a p-v diagram.
Answers: 2

Physics, 22.06.2019 18:00
Which is the most accurate name for the ionic compound cas?
Answers: 1

You know the right answer?
What is the enthalpy change (in kj) of a chemical reaction that raises the temperature of 250.0 ml o...
Questions

Mathematics, 23.02.2021 07:40

Mathematics, 23.02.2021 07:40

Chemistry, 23.02.2021 07:40



Mathematics, 23.02.2021 07:40

English, 23.02.2021 07:40



Mathematics, 23.02.2021 07:40


English, 23.02.2021 07:40


Mathematics, 23.02.2021 07:40


History, 23.02.2021 07:40

Mathematics, 23.02.2021 07:40

Mathematics, 23.02.2021 07:40


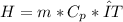
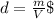 ⇒
⇒ 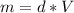
![m=250[mL]*1,25[\frac{g}{mL}]=312,5 [g]](/tpl/images/0222/9306/18a07.png)
![H=312,5*3,74*(-3,33)=-3891 [J]=-3,81[kJ]](/tpl/images/0222/9306/684a5.png)


