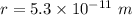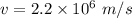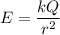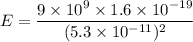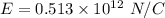Physics, 04.09.2019 18:20 isaiahbjohnson1839
In the bohr model of the hydrogen atom, the electron moves in a circular orbit of radius 5.3 x 10^-11 m with a speed of 2.2 x 10^6 m/s.
if we are viewing the atom in such a way that the electron's orbit is in the plane of the paper with the electron moving clockwise, find the magnitude of the electric field that the electron produces at the location of the nucleus (treated as a point).

Answers: 2


Another question on Physics

Physics, 21.06.2019 16:30
Dalton’s model of an atom is the best described as a) a solar system b) a solid sphere c) a cloud d) a scoop of chocolate chip ice cream
Answers: 1

Physics, 22.06.2019 05:20
Suppose an objects initial velocity is 10m/s and it’s final velocity is 4 m/s. mass is constant. what can best be concluded about the object based in the work-energy theorem
Answers: 2

Physics, 22.06.2019 08:50
The experiment was repeated many years later but the gases were mixed in a different type of container. a white solid was obtained which was xenon fluoride. predict whether you think 1) krypton and 2) radon will react with fluorine. explain reasons for your predictions
Answers: 3

Physics, 22.06.2019 15:30
This is the number of complete movements of a wave per second.
Answers: 1
You know the right answer?
In the bohr model of the hydrogen atom, the electron moves in a circular orbit of radius 5.3 x 10^-1...
Questions



Mathematics, 27.05.2021 23:50





Mathematics, 27.05.2021 23:50

Mathematics, 27.05.2021 23:50

Mathematics, 27.05.2021 23:50

Physics, 27.05.2021 23:50


Arts, 27.05.2021 23:50

Biology, 27.05.2021 23:50

English, 27.05.2021 23:50





History, 27.05.2021 23:50

 .
.