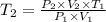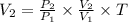Physics, 03.09.2019 00:30 zahradawkins2007
Asample of a monatomic ideal gas initially at temperature t, undergoes a process during which the pressure of the gas triples and its volume also triples. what is the change in the temperature (δt) of the gas, in kelvins? (a)t (b) 8t (c) 9t (d) t/9 (e) t/8

Answers: 3


Another question on Physics

Physics, 22.06.2019 06:30
In positive numbers less than 1, the zeros between the decimal point and a non-zero number are blank significant
Answers: 1

Physics, 22.06.2019 07:50
Calculate the ratio of h+ ions to oh– ions at a ph = 6. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 6. are they the same? why or why not? record your explanation in table a. what is the concentration of h+ ions at a ph = 6? mol/l what is the concentration of oh– ions at a ph = 6? mol/l what is the ratio of h+ ions to oh– ions at a ph = 6? : 1
Answers: 1

Physics, 22.06.2019 14:30
Two steel balls, each of mass m, are welded to a light rod of length l and negligible mass and are initially at rest on a smooth horizontal surface. a horizontal force of magnitude f is suddenly applied to the rod as shown. determine (a) the instantaneous acceleration a of the mass center g and (b) the corresponding rate at which the angular velocity of the assembly about g is changing with time.
Answers: 2

Physics, 22.06.2019 15:30
What is the increase in density of a medium due to wave travel?
Answers: 2
You know the right answer?
Asample of a monatomic ideal gas initially at temperature t, undergoes a process during which the pr...
Questions



Chemistry, 23.10.2020 19:20

Health, 23.10.2020 19:20

Social Studies, 23.10.2020 19:20

Spanish, 23.10.2020 19:20

Biology, 23.10.2020 19:20

Biology, 23.10.2020 19:20

Mathematics, 23.10.2020 19:20

Social Studies, 23.10.2020 19:20

Mathematics, 23.10.2020 19:20

Health, 23.10.2020 19:20



Mathematics, 23.10.2020 19:20



English, 23.10.2020 19:20