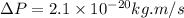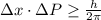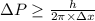Rutherford's scattering experiments gave the first indications that an atom consists of a small, dense, positively charged nucleus surrounded by negatively charged electrons. his experiments also allowed for a rough determination of the size of the nucleus. in this problem, you will use the uncertainty principle to get a rough idea of the kinetic energy of a particle inside the nucleus. consider a nucleus with a diameter of roughly 5.0×10^−15 meters. consider a particle inside the nucleus. the uncertainty δx in its position is equal to the diameter of the nucleus. what is the uncertainty δp of its momentum? to find this, use δx δp ≥ h

Answers: 3


Another question on Physics

Physics, 22.06.2019 12:00
In an experiment, how can i change human errors? be specific.
Answers: 1

Physics, 22.06.2019 13:40
What is the thinnest soap film (excluding the case of zero thickness) that appears black when illuminated with light with a wavelength of 480 ? the index of refraction of the film is 1.34, and there is air on both sides of the film. express your answer in nanometers. hint 1. how to approach th
Answers: 1

Physics, 22.06.2019 16:20
What is the single most important equation in all of physics?
Answers: 1

Physics, 22.06.2019 23:40
A2.50 × 105 w motor is used for 26.4 s to pull a boat straight toward shore. how far does the boat move toward shore if a force of 4.20 × 104 n is applied by the motor?
Answers: 2
You know the right answer?
Rutherford's scattering experiments gave the first indications that an atom consists of a small, den...
Questions

History, 01.02.2021 19:10



Mathematics, 01.02.2021 19:10




Mathematics, 01.02.2021 19:10



Mathematics, 01.02.2021 19:10


Mathematics, 01.02.2021 19:10


Advanced Placement (AP), 01.02.2021 19:10

Health, 01.02.2021 19:10

English, 01.02.2021 19:10

Mathematics, 01.02.2021 19:10