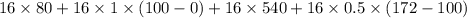16 grams of ice at –32°c is to be changed to steam at 172°c. the entire process requires cal. round your answer to the nearest whole number. the specific heat of both ice and steam is 0.5 cal/g°c. the specific heat of water is 1.00 cal/gk. the heat of fusion is 80 cal/g and the heat of vaporization is 540 cal/g.

Answers: 3


Another question on Physics

Physics, 21.06.2019 13:30
The mentor is usually a peer of the individual that acts as a role model and the individual get started in adult life.
Answers: 3

Physics, 21.06.2019 14:20
The eel has a certain amount of rotational kinetic energy when spinning at 14 spins per second. if it swam in a straight line instead, about how fast would the eel have to swim to have the same amount of kinetic energy as when it is spinning?
Answers: 1

Physics, 21.06.2019 22:50
If the temperature were raised very high, classically what would we expect the heat capacity per object to be for this one-dimensional system? give a numerical value. chigh t = __ j/k/object (one reason for the discrepancy is that the high-temperature limit assumes that the number of oscillators is large (n > > 1), which is not the case in this tiny system.)
Answers: 2

Physics, 21.06.2019 23:30
Which of the following statements is true about women and minorities in early psychology? a. in the early 20th century, numerous graduate schools recruited women and minorities to study psychology. b. opportunities in higher education were limited for women and minorities in the early 20th century due to discrimination. c. despite some obstacles, there were numerous employment opportunities for women and minorities. d. women and minorities were often selected to do academic research in an effort to make the field more diverse.
Answers: 3
You know the right answer?
16 grams of ice at –32°c is to be changed to steam at 172°c. the entire process requires cal. round...
Questions


Mathematics, 01.07.2019 21:30


English, 01.07.2019 21:30


Arts, 01.07.2019 21:30






History, 01.07.2019 21:30


Computers and Technology, 01.07.2019 21:30


Biology, 01.07.2019 21:30




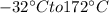
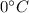 then change its state to water and then raises water temperature from 0 to
then change its state to water and then raises water temperature from 0 to 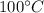 .
.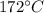
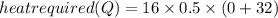 +
+