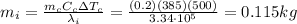Physics, 05.08.2019 01:10 katherineweightman
A200.0-g copper cylinder at 500°c is placed on a large block of ice at 0.00°c. assume that no energy is transferred to the surroundings.
what is the mass of the ice that will melt?
the specific heat of copper is 385 j/kg•°c.

Answers: 1


Another question on Physics


Physics, 22.06.2019 11:10
While standing outdoors one evening, you are exposed to the following four types of electromagnetic radiation: yellow light from a sodium street lamp, radio waves from an am radio station, radio waves from an fm radio station, and microwaves from an antenna of a communications system. rank these type of waves in terms of increasing photon energy.
Answers: 3

Physics, 22.06.2019 14:00
Explain why you think this diagram shows what happened to the carbon in the biodome.
Answers: 2

Physics, 22.06.2019 16:20
What is the mass of the water that is being heated? it requires 2,500 joules to raise a certain amount of water (c = 4.186 jig c) from 20.0°c to 60.0°c. o 159 o 40 g o 63 g o 80 g
Answers: 2
You know the right answer?
A200.0-g copper cylinder at 500°c is placed on a large block of ice at 0.00°c. assume that no energy...
Questions

Mathematics, 07.06.2021 17:30

Mathematics, 07.06.2021 17:30

Mathematics, 07.06.2021 17:30



Mathematics, 07.06.2021 17:30

Mathematics, 07.06.2021 17:30


Mathematics, 07.06.2021 17:30

Mathematics, 07.06.2021 17:30

Mathematics, 07.06.2021 17:30






English, 07.06.2021 17:30

Arts, 07.06.2021 17:30

Physics, 07.06.2021 17:30

Mathematics, 07.06.2021 17:30

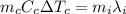
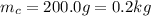 is the mass of the copper
is the mass of the copper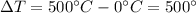 is the change in temperature of the copper (the copper stops to give heat to the ice when they are in thermal equilibrium, so when they have reached the same temperature)
is the change in temperature of the copper (the copper stops to give heat to the ice when they are in thermal equilibrium, so when they have reached the same temperature)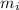 is the mass of ice
is the mass of ice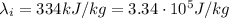 is the latent heat of fusion of ice
is the latent heat of fusion of ice