Physics, 02.08.2019 18:10 babyface1686
At 25°, the free energy of formation of gaseous water is -229 kj/mol. calculate δg for the following reaction if the hydrogen is supplied at 4.00 atm and the oxygen is supplied at 3.00 atm, while the water produced is at 1.00 atm pressure.

Answers: 3


Another question on Physics

Physics, 21.06.2019 21:50
Acar travels along a highway with a velocity of 24 m/s, west. the car exits the highway; and 4.0 s later, its instantaneous velocity is 16 m/s, 45° north of west. what is the magnitude of the average acceleration of the car during the four-second interval?
Answers: 2

Physics, 21.06.2019 22:50
Calculate the heat capacity per object when the total energy is 4 quanta. (think of this in terms of increasing from 3.5 quanta of energy in the system to 4.5 quanta of energy in the system, if that were possible.) c4 = __ j/k/object
Answers: 2

Physics, 22.06.2019 04:10
Calculate the work done by an external agent during an isothermal compression of 1.00 mol of oxygen from a volume of 22.4 l at 10∘c and 1.0 atm pressure to 16.8l
Answers: 2

Physics, 22.06.2019 11:50
Select all that applywhat are some basic resources a family is expected to provide for children? educationclothesspending
Answers: 2
You know the right answer?
At 25°, the free energy of formation of gaseous water is -229 kj/mol. calculate δg for the following...
Questions

Mathematics, 12.09.2021 20:20


Computers and Technology, 12.09.2021 20:20







Biology, 12.09.2021 20:20

Mathematics, 12.09.2021 20:20

History, 12.09.2021 20:20

Social Studies, 12.09.2021 20:20

Chemistry, 12.09.2021 20:20


Chemistry, 12.09.2021 20:20

English, 12.09.2021 20:20

Mathematics, 12.09.2021 20:20

Engineering, 12.09.2021 20:20

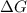 for the reaction is -467.595 kJ/mol
for the reaction is -467.595 kJ/mol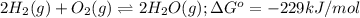
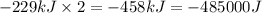 (Conversion factor: 1kJ = 1000J)
(Conversion factor: 1kJ = 1000J)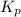 for the given reaction:
for the given reaction: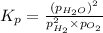
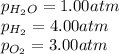
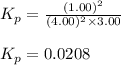
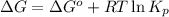
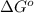 = Standard Gibbs' free energy change of the reaction = -458000 J
= Standard Gibbs' free energy change of the reaction = -458000 J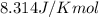
![25^oC=[25+273]K=298K](/tpl/images/0162/6752/df1f6.png)