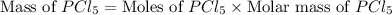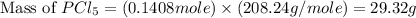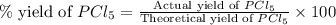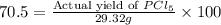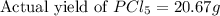Phosphorous reacts with chlorine gas to produce phosphorous pentachloride. calculate the mass of product produced when 25.0 g of phosphorous reacts with 25.0 grams of chlorine. calculate the mass of product produced if the reaction occurred with a 70.5 percent yield.

Answers: 1


Another question on Physics

Physics, 21.06.2019 21:10
State one advantage and one disadvantage of using a plane mirrior as a driving mirrior
Answers: 1

Physics, 22.06.2019 16:30
An astronaut in space cannot use a scale or balance to weigh objects because there is no gravity. but she does have devices to measure distance and time accurately. she knows her own mass is 77.4 kg , but she is unsure of the mass of a large gas canister in the airless rocket. when this canister is approaching her at 3.50 m/s , she pushes against it, which slows it down to 1.30 m/s (but does not reverse it) and gives her a speed of 2.60 m/s . what is the mass of the canister?
Answers: 1

Physics, 22.06.2019 18:00
Gabby calls her cousin who lives in a different state and tells her to turn her radio to channel 98.7 so they can listen to their favorite song that is playing. her cousin turns her radio to 98.7, but does not hear the same song gabby hears. which most likely explains why?
Answers: 1

Physics, 22.06.2019 18:00
According to newton’s law of universal gravitation, which statements are true? as we move to higher altitudes, the force of gravity on us decreases. as we move to higher altitudes, the force of gravity on us increases. as we gain mass, the force of gravity on us decreases. as we gain mass, the force of gravity on us increases. as we move faster, the force of gravity on us increases.
Answers: 2
You know the right answer?
Phosphorous reacts with chlorine gas to produce phosphorous pentachloride. calculate the mass of pro...
Questions



History, 31.07.2019 05:30








Health, 31.07.2019 05:30

Mathematics, 31.07.2019 05:30

Social Studies, 31.07.2019 05:30


Mathematics, 31.07.2019 05:30





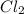 = 25 g
= 25 g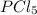 = 208.24 g/mole
= 208.24 g/mole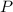 and
and 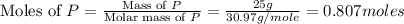
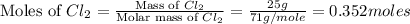
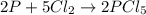
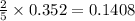 moles of
moles of