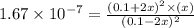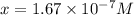Physics, 25.07.2019 00:20 mendezmarco2004
2h2s(g)⇌2h2(g)+s2(g),kc=1.67×10−7 at 800∘c is carried out at the same temperature with the following initial concentrations: [h2s]=0.100m, [h2]=0.100m, and [s2]=0.00 m. find the equilibrium concentration of s2. express the molarity to three significant figures.

Answers: 1


Another question on Physics

Physics, 22.06.2019 15:00
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation 2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!
Answers: 3

Physics, 22.06.2019 16:20
Amagnetic field applies forces on: a)static chargesb)moving chargesc)water flow
Answers: 1

Physics, 22.06.2019 17:00
How much energy is supplied to each coulomb of charge that flows through a 12-v battery?
Answers: 1

Physics, 22.06.2019 18:30
How many meters will a car travel if its speed is 45 m/s in an interval of 11 seconds? question 2 options: a) 450 meters b) 495 meters c) 4.09 meters d) 498 meters
Answers: 2
You know the right answer?
2h2s(g)⇌2h2(g)+s2(g),kc=1.67×10−7 at 800∘c is carried out at the same temperature with the following...
Questions







Mathematics, 23.07.2019 14:30


Mathematics, 23.07.2019 14:30


History, 23.07.2019 14:30


History, 23.07.2019 14:30

English, 23.07.2019 14:30

Health, 23.07.2019 14:30


Computers and Technology, 23.07.2019 14:30


Mathematics, 23.07.2019 14:30

Spanish, 23.07.2019 14:30

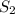 at equilibrium will be,
at equilibrium will be, 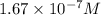
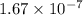
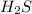 = 0.100 M
= 0.100 M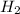 = 0.100 M
= 0.100 M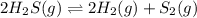
![K_c=\frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/0129/1468/3ac5e.png)