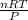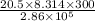Physics, 20.07.2019 02:20 khalilh1206
Acylinder with a movable piston contains 20.5 moles of a monatomic ideal gas at a pressure of 2.86 × 105 pa. the gas is initially at a temperature of 300 k. an electric heater adds 58600 j of energy into the gas while the piston moves in such a way that the pressure remains constant. it may you to recall that cp = 20.79 j/k/mole for a monatomic ideal gas, and that the number of gas molecules is equal to avagadros number (6.022 × 1023) times the number of moles of the gas. what is the temperature of the gas after the energy is added?

Answers: 1


Another question on Physics

Physics, 22.06.2019 10:10
In a simplified model of the human eye, the aqueous and vitreous humors and the lens all have a refractive index of 1.40, and all the refraction occurs at the cornea, whose vertex is 2.60 cm from the retina. what should be the radius of curvature of the cornea such that the image of an object 40.0 cm from the cornea’s vertex is focused on the retina?
Answers: 1

Physics, 22.06.2019 17:30
Which of the choices are parts of an atom? select all that apply. a.) compound b.) electron c.) neutron d.) molecule e.) photon f.) proton g.) element
Answers: 1

Physics, 22.06.2019 22:00
Use 4 to 5 complete sentences, explain the concepts of beats and how beats are produces.
Answers: 2

Physics, 23.06.2019 04:10
Acar traveling at a constant speed of 22.0 m/s passes a trooper hidden behind a billboard. one second after the speeding car passes the billboard, the trooper sets off in chase with a constant acceleration of 3.50 m/s2. how long does it take the trooper to overtake the speeding car? solve this problem by a graphical method. on the same graph, plot position versus time for the car and the trooper.
Answers: 2
You know the right answer?
Acylinder with a movable piston contains 20.5 moles of a monatomic ideal gas at a pressure of 2.86 ×...
Questions

Mathematics, 21.05.2020 23:14

English, 21.05.2020 23:14





Computers and Technology, 21.05.2020 23:14



Mathematics, 21.05.2020 23:14


Mathematics, 21.05.2020 23:14

English, 21.05.2020 23:14



Mathematics, 21.05.2020 23:14




Mathematics, 21.05.2020 23:14