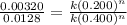Answers: 1


Another question on Physics

Physics, 22.06.2019 03:50
Three different objects, all with different masses, are initially at rest at the bottom of a set of steps. each step is of uniform height . the mass of each object is a multiple of the base mass : object 1 has mass 4..00m , object 2 has mass 1..96m , and object 3 has mass . when the objects are at the bottom of the steps, define the total gravitational potential energy of the three-object system to be zero. if the objects are then relocated as shown, what is the new total potential energy of the system? each answer requires the numerical coefficient to an algebraic expression. each algebraic expression is given using some combination of the variables , , and , where is the acceleration due to gravity. enter only the numerical coefficient. (example: if the answer is 1..23mgd , just enter 1.23)
Answers: 3


Physics, 22.06.2019 08:50
You are given a vector a = 125i and an unknown vector b that is perpendicular to a. the cross-product of these two vectors is a × b = 98k. what is the y-component of vector b?
Answers: 1

Physics, 22.06.2019 14:00
Select for each of the following statements whether it is correct or incorrect. (a) in an isothermal expansion of an ideal gas. (b) the temperature remains constant. (b) the pressure remains constant. (c) there is work done by the gas. (d) there is heat added to the gas. (e) the change in internal energy equals zero.
Answers: 1
You know the right answer?
Consider the following initial rate data for the decomposition of ab to yield a and b: [ab], mol/l...
Questions

Chemistry, 10.12.2019 06:31


Physics, 10.12.2019 06:31


History, 10.12.2019 06:31

Mathematics, 10.12.2019 06:31

Mathematics, 10.12.2019 06:31

Social Studies, 10.12.2019 06:31

History, 10.12.2019 06:31

Mathematics, 10.12.2019 06:31










![R=k[AB]^2](/tpl/images/0106/2067/35d31.png)
![R=k[AB]^n](/tpl/images/0106/2067/249d7.png)
![R_1=k[AB_1]^n](/tpl/images/0106/2067/f1794.png)
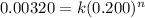 .............(1)
.............(1)![R_2=k[AB_2]^n](/tpl/images/0106/2067/bf2e9.png)
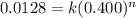 .............(2)
.............(2)