Answers: 2


Another question on Physics

Physics, 21.06.2019 19:20
How would you place two electrons on a sphere of radius r so that the electrostatic potential energy is minimized. how would you place three electrons to solve the same problem.
Answers: 1

Physics, 21.06.2019 21:50
When applying kirchhoff's rules, one of the essential steps is to mark each resistor with plus and minus signs to label how the electric potential changes from one end of the resistor to the other. the circuit in the drawing contains four resistors, each marked with the associated plus and minus signs. however, one resistor is marked incorrectly. which one is it?
Answers: 1

Physics, 21.06.2019 23:50
Any color picture tube and electric field exerts a net force of magnitude 1.68x 10^-13 n on an electron the rest mass of an electron is 9.11 x 10^-13 what is the electron acceleration?
Answers: 3

Physics, 22.06.2019 07:00
Aball has an initial velocity of 3 m/s. if there is no friction, what is the highest it could roll?
Answers: 1
You know the right answer?
The frequency factor and activation energy for a chemical reaction are a = 4.23 x 10–12 cm3/(molecul...
Questions


Mathematics, 20.11.2020 21:50

Chemistry, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50



Mathematics, 20.11.2020 21:50

Computers and Technology, 20.11.2020 21:50


History, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50



Spanish, 20.11.2020 21:50


Chemistry, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50

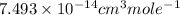
![k=A\times e^{[\frac{-Ea}{RT}]}](/tpl/images/0105/9531/eef14.png)
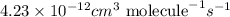
![k=4.23\times 10^{-12}cm^3\text{ molecule}^{-1}s^{-1}\times e^{[\frac{-12.9kJ/mol}{(8.314J/mole.K)\times (384.7K)}]}](/tpl/images/0105/9531/82c0d.png)