
Physics, 05.07.2019 03:20 0Brittany0
Suppose 8.50 ✕ 10^5 j of energy are transferred to 1.63 kg of ice at 0°c. the latent heat of fusion and specific heat of water are lf = 3.33 ✕ 105 j/kg and c = 4186 j (kg · °c) . hint (a) calculate the energy (in j) required to melt all the ice into liquid water. (enter your answer to at least three significant figures.) j (b) how much energy (in j) remains to raise the temperature of the liquid water? (enter your answer to at least three significant figures.) j (c) determine the final temperature of the liquid water in celsius. °c

Answers: 3


Another question on Physics



Physics, 22.06.2019 07:00
The seven sisters, seven stars located more than 400 light-years away in the taurus constellation can be seen here with venus and another constellation, orion. the seven stars stay grouped together but seem to move, like the planet venus. why do stars seem to move like planets in the night sky?
Answers: 3

Physics, 22.06.2019 09:00
Inside of a windmill or a dam is a that is used to transform one type of energy into electrical energy. a. motor b. generator c. transformer d. power pack
Answers: 2
You know the right answer?
Suppose 8.50 ✕ 10^5 j of energy are transferred to 1.63 kg of ice at 0°c. the latent heat of fusion...
Questions




















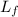 = Latent heat of fusion of ice to water = 3.33 x 10⁵ J/kg
= Latent heat of fusion of ice to water = 3.33 x 10⁵ J/kg 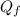 = Energy required to melt the ice
= Energy required to melt the ice 

