
Physics, 03.07.2019 23:30 itaheart101
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorimeter at a temperature of 22°c. determine the final temperature of the system consisting of the ice, water, and calorimeter. remember that the ice must first warm to 0°c, melt, and then continue warming as water. the specific heat of ice is 0.500 cal/g·°c = 2090 j/kg°c.

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:30
Two identical 82 mg dust particles very far apart (pee 0) are moving directly toward each other at a speed of 3698 m/s. the charge on each is-719 ? c. determine how close they will get to each other. let k = 9x109 n-m2/c2 & ignore gravity.
Answers: 1

Physics, 22.06.2019 09:30
The necleus of an atom is made up of what subatomic particles?
Answers: 1

Physics, 22.06.2019 10:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 3

Physics, 22.06.2019 21:30
The diagram shows a boulder rolling down a hill into a valley and then up the opposite hill. at which position does the boulder have the greatest kinetic energy? a b c d
Answers: 2
You know the right answer?
A40 g block of ice is cooled to -69°c and is then added to 590 g of water in an 80 g copper calorime...
Questions


History, 19.10.2019 06:00

Mathematics, 19.10.2019 06:00


Mathematics, 19.10.2019 06:00



History, 19.10.2019 06:00

Chemistry, 19.10.2019 06:00

Spanish, 19.10.2019 06:00

History, 19.10.2019 06:00

Biology, 19.10.2019 06:00



Business, 19.10.2019 06:00

English, 19.10.2019 06:00



History, 19.10.2019 06:00

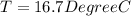
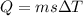
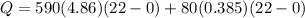
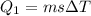
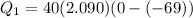
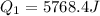
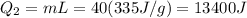
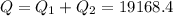
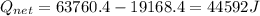
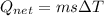
![44592 = (590 + 40)(4.186)(T - 0) + 80(0.385)(T - 0)[tex]T = 16.7 Degree C](/tpl/images/0048/1109/c6b32.png)


