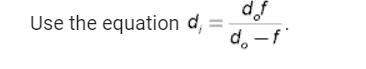Physics, 22.06.2019 13:00 mandilynn22
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:00
What is work? how much work is done to move an object 0 meters?
Answers: 1


Physics, 22.06.2019 13:40
Dao makes a table to identify the variables used in the equations for centripetal acceleration. what quantities belong in cells x and y?
Answers: 2

Physics, 22.06.2019 14:30
What was the first instrument to ever record an earthquake?
Answers: 1
You know the right answer?
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused th...
Questions


Business, 22.01.2021 06:00

Mathematics, 22.01.2021 06:00


Mathematics, 22.01.2021 06:00




English, 22.01.2021 06:00






History, 22.01.2021 06:00




Mathematics, 22.01.2021 06:00