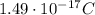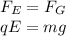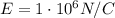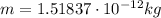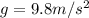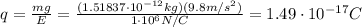Physics, 26.01.2020 23:31 moneybabyy38
In 1909 robert millikan was the first to find the charge of an electron in his now-famous oil drop experiment. in the experiment tiny oil drops are sprayed into a uniform electric field between a horizontal pair of oppositely charged plates. the drops are observed with a magnifying eyepiece, and the electric field is adjusted so that the upward force q e on some negatively charged oil drops is just sufficient to balance the downward force m g of gravity. millikan accurately measured the charges on many oil drops and found the values to be whole-number multiples of 1.6 × 10−19 c — the charge of the electron. for this he won the nobel prize. if a drop of mass 1.51837 × 10−12 kg remains stationary in an electric field of 1 × 106 n/c, what is the charge on this drop? the acceleration due to gravity is 9.8 m/s 2 . answer in units of c.

Answers: 2


Another question on Physics

Physics, 21.06.2019 14:30
How much work does the charge escalator do to move 2.30 μc of charge from the negative terminal to the positive terminal of a 3.00 v battery?
Answers: 1

Physics, 22.06.2019 05:00
Which is the best predictor of the radioactive nature of an isotope? o the proton-to-electron ratio the neutron-to-proton ratio o the neutron-to-electron ratio the electron-to-proton ratio
Answers: 1

Physics, 22.06.2019 15:30
How many neutrons does element x if it’s atomic number is 28 and its mass number is 87
Answers: 1

Physics, 23.06.2019 03:00
The device shows the relative humidity at 22°c. what’s the water vapor density if the maximum water vapor in air at this temperature is 20 grams/cubic meter? a device showing that at 22 degrees celsius the relative humidity is 58%. a. 11.6% b. 11.6 g/m3 c. 12.76% d. 12.76 g/m3 reset next
Answers: 2
You know the right answer?
In 1909 robert millikan was the first to find the charge of an electron in his now-famous oil drop e...
Questions





History, 07.11.2020 03:00


Biology, 07.11.2020 03:00

Computers and Technology, 07.11.2020 03:00




Spanish, 07.11.2020 03:00




Mathematics, 07.11.2020 03:00