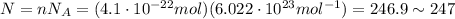Physics, 18.10.2019 14:30 dianepowers1
The lowest pressure attainable using the best available vacuum techniques is about 10^−12 n/m^2.
at such a pressure, how many molecules are there per cm^3 at 19 °c?

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:40
Ablock of mass m = 2.5 kg is attached to a spring with spring constant k = 710 n/m. it is initially at rest on an inclined plane that is at an angle of θ = 23° with respect to the horizontal, and the coefficient of kinetic friction between the block and the plane is μk = 0.19. in the initial position, where the spring is compressed by a distance of d = 0.16 m, the mass is at its lowest position and the spring is compressed the maximum amount. take the initial gravitational energy of the block as zero. a) what is the block's initial mechanical energy? b) if the spring pushes the block up the incline, what distance, l, in meters will the block travel before coming to rest? the spring remains attached to both the block and the fixed wall throughout its motion.
Answers: 3

Physics, 22.06.2019 07:00
Critical mass is the of material required to produce a chain reaction. a.) minimum amount b.) atomic mass c.) precise amount d.) maximum amount
Answers: 1

Physics, 22.06.2019 08:30
Does anyone know how to solve this problem? i really need . i made an attempt but i just cant get it. a metal rod is 25.000 cm long at 25.0 degrees celsius. when heated to 102.0 degrees celsius, it is 25.054 cm long. what is the coefficient of linear expansion for this metal.
Answers: 3

Physics, 22.06.2019 10:30
The precision of a laboratory instrument is ± 0.05 g. the accepted value for your measurement is 7.92 g. which measurements are in the accepted range? check all that apply. 7.85 g 7.89 g 7.91 g 7.97 g 7.99 g
Answers: 1
You know the right answer?
The lowest pressure attainable using the best available vacuum techniques is about 10^−12 n/m^2.
Questions

Social Studies, 11.06.2020 19:57


Mathematics, 11.06.2020 19:57












Mathematics, 11.06.2020 19:57





History, 11.06.2020 19:57

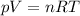
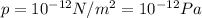 is the lowest pressure attainable
is the lowest pressure attainable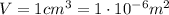 is the volume we are considering
is the volume we are considering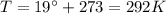 is the absolute temperature
is the absolute temperature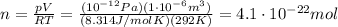
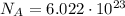 (avogadro number)
(avogadro number)