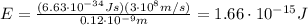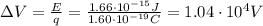Through what potential difference δv must electrons be accelerated (from rest) so that they will have the same wavelength as an x-ray of wavelength 0.120 nm ? use 6.63×10−34 j⋅s for planck's constant, 9.11×10−31 kg for the mass of an electron, and 1.60×10−19 c for the charge on an electron. express your answer using three significant figures. view available hint(s) δv δ v deltav = 104 v submitprevious answers correct significant figures feedback: your answer 104.7 v was either rounded differently or used a different number of significant figures than required for this part. part b through what potential difference δv must electrons be accelerated so they will have the same energy as the x-ray in part a? use 6.63×10−34 j⋅s for planck's constant, 3.00×108 m/s for the speed of light in a vacuum, and 1.60×10−19 c for the charge on an electron. express your answer using three significant figures.

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:40
Argon is adiabatically compressed from an initial volume of 16 liters to a final volume of 2 liters. by what factor do the following quantities change? do they increase or decrease? (a) the rms speed (b) the thermal energy of the gas (c) the molar specific heat cv (d) the pressure
Answers: 3

Physics, 22.06.2019 05:40
An ideal polarizer with its transmission axis rotated 30 degrees to the vertical is placed in a beam of unpolarized light of intensity 10w/m^2. after passing through the polarizer, what is the intensity of the beam? a. 8.7 w/m^2 b. 7.5 w/m^2 c. 5.0 w/m^2 d. 10 w/m^2 e. 2.5 w/m^2
Answers: 1

Physics, 22.06.2019 06:10
Which transition by an electron will release the greatest amount of energy? oa ob oc od
Answers: 2

Physics, 22.06.2019 14:30
Which compound is held together by the electrostatic force between two ions? a. co2 b. cci4 c. h2s d. mgf2
Answers: 1
You know the right answer?
Through what potential difference δv must electrons be accelerated (from rest) so that they will hav...
Questions




English, 02.07.2019 15:40




Mathematics, 02.07.2019 15:40


Social Studies, 02.07.2019 15:40


Computers and Technology, 02.07.2019 15:40

Physics, 02.07.2019 15:40

Mathematics, 02.07.2019 15:40



Social Studies, 02.07.2019 15:50


Mathematics, 02.07.2019 15:50

History, 02.07.2019 15:50

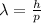
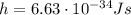 is the Planck constant
is the Planck constant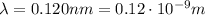
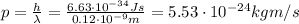
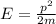
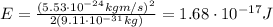
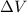 is
is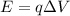
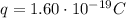 is the electron charge
is the electron charge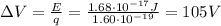
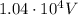
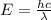
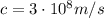 is the speed of light
is the speed of light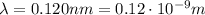 is the wavelength
is the wavelength