Which compound has the highest boiling point? explain your reasoning. ch4 or ch3ch3
que...

Physics, 16.10.2019 01:30 cthompson1107
Which compound has the highest boiling point? explain your reasoning. ch4 or ch3ch3
question 28 options:
ch3ch3. it has a greater mass and number of electrons which causes greater dispersion forces and a higher boiling point.
ch4 has stronger dispersion forces compared to ch3ch3 which leads to a higher boiling point.
ch3ch3. it is flatter which causes greater dispersion forces than ch4.
ch4 has a lower mass compared to ch3ch3 which leads to a higher boiling point.

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:30
11. you want to calculate the displacement of an object thrown over a bridge. using -10 m/s2 for acceleration due to gravity, what would be the total displacement of the object if it took 8 seconds before hitting the water?
Answers: 1

Physics, 21.06.2019 20:50
Aball is thrown upward in the air, and its height above the ground after t seconds is h ( t ) = 57 t − 16 t 2 feet. find the time t when the ball will be traveling upward at 14.25 feet per second.
Answers: 1

Physics, 22.06.2019 02:20
According to newton’s first law of motion, which force is expected to cause a body to accelerate?
Answers: 1

You know the right answer?
Questions



English, 07.07.2021 22:30


Mathematics, 07.07.2021 22:30

Social Studies, 07.07.2021 22:30

Mathematics, 07.07.2021 22:30






World Languages, 07.07.2021 22:30

English, 07.07.2021 22:30



Mathematics, 07.07.2021 22:40



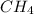 has a lower mass compared to
has a lower mass compared to 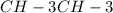 which leads to a higher boiling point.
which leads to a higher boiling point.

