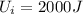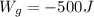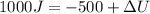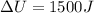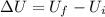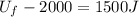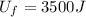Physics, 22.06.2019 15:00 tinagibbs98
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation 2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!

Answers: 3


Another question on Physics

Physics, 21.06.2019 20:30
Air enters a compressor operating at steady state at 1.05 bar, 300 k, with a volumetric flow rate of 93 m3/min and exits at 12 bar, 400 k. heat transfer occurs at a rate of 15.5 kw from the compressor to its surroundings. assuming the ideal gas model for air neglecting kinetic potential energy effects, determine the power in put in kw.
Answers: 2

Physics, 22.06.2019 02:40
What happens when chlorine reacts with bromine? a. electrons move from the chlorine atoms to the bromine atoms. b. electrons move from the bromine atoms to the chlorine atoms. c. electrons are shared between the chlorine atoms and the bromine atoms. d. electrons become delocalized among the atoms.
Answers: 2

Physics, 22.06.2019 04:00
All the simple machines make work easier to do by changing the or of a force. a. size; type b. work; type c. size; direction d. type; direction
Answers: 2

Physics, 22.06.2019 14:00
Why is rain likely when warm, moisture-laden air meets cold air? a) the lighter warm air will rise and cool down, causing condensation and rain. b) the cold air moves faster and pushes the warm air away, causing condensation and rain. c) the moisture in the warm air condenses on contact with the cold air, causing rain to fall. d) the cold air mixes with the warm air, reducing its temperature causing moisture to condense.
Answers: 1
You know the right answer?
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the pist...
Questions

History, 26.03.2020 23:22

English, 26.03.2020 23:22







History, 26.03.2020 23:23

English, 26.03.2020 23:23

History, 26.03.2020 23:23

Spanish, 26.03.2020 23:23

Mathematics, 26.03.2020 23:23







Mathematics, 26.03.2020 23:23

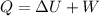
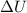 = change in internal energy of the system
= change in internal energy of the system