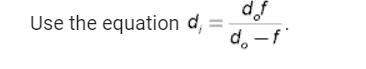Physics, 02.07.2019 03:30 avahrhey24
Select all of the statements that are true. each orbit holds a fixed number of electrons. the n = 1 orbit can only hold two electrons. each orbit can hold an unlimited number of electrons. each orbit is limited to a maximum of four electrons.

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:00
Find the momentum for a 2.6-kg brick parachuting straight downward at a constant speed of 8.4 m/s .
Answers: 3

Physics, 22.06.2019 00:30
Aball tossed vertically upward from the ground next to a building passes the bottom of a window 1.8 s after being tossed and passes the top of the window 0.20 s later. the window is 2.0 m high from top to bottom. what was the ball's initial velocity? the unit vector j^ is directed upward. how far is the bottom of the window from the launch position? how high does the ball rise above the launch position?
Answers: 1


Physics, 22.06.2019 02:50
Steam is generated in a boiler of a cogeneration plant at 10 mpa and 450°c at a steady rate of 5 kg/s. in normal operation, steam expands in a turbine to a pressure of 0.5 mpa and is then routed to the process heater, where it supplies the process heat. steam leaves the process heater as a saturated liquid and is pumped to the boiler pressure. in this mode, no steam passes through a condenser, which operates at 20 kpa. (a) determine the power produced in the turbine and the rate at which process heat is supplied in this mode. (b) determine the power produced in the turbine and the rate of process heat supplied if only 60 percent of the steam is routed to the process heater and the remainder is expanded to the condenser pressure. (3.32 mw; 9.69 mw; 4.25 mw; 5.82 mw)
Answers: 3
You know the right answer?
Select all of the statements that are true. each orbit holds a fixed number of electrons. the n = 1...
Questions

Mathematics, 30.05.2020 03:04

Mathematics, 30.05.2020 03:04




Computers and Technology, 30.05.2020 03:04



Biology, 30.05.2020 03:04

History, 30.05.2020 03:04

Biology, 30.05.2020 03:04




History, 30.05.2020 03:04

Mathematics, 30.05.2020 03:04




Mathematics, 30.05.2020 03:04

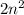 , where n is the number of the orbit. For instance, when n=1 it means
, where n is the number of the orbit. For instance, when n=1 it means 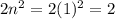 . This particular orbit can only hold up to two electrons. Even though the electrons can gain energy and move to higher orbits or electrons from higher orbits can lose energy and drop to the n=1 level, the energy level would not allow more electrons to enter the orbit once it is full. Again the octet rule, which states that atoms achieve stability by having 8 valence electrons, limits the maximum number of electrons that can be occupied by an orbit. The gain and loss of electrons is done to achieve the noble gas configuration and once that is reached no more electron can be added to an orbit
. This particular orbit can only hold up to two electrons. Even though the electrons can gain energy and move to higher orbits or electrons from higher orbits can lose energy and drop to the n=1 level, the energy level would not allow more electrons to enter the orbit once it is full. Again the octet rule, which states that atoms achieve stability by having 8 valence electrons, limits the maximum number of electrons that can be occupied by an orbit. The gain and loss of electrons is done to achieve the noble gas configuration and once that is reached no more electron can be added to an orbit