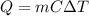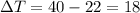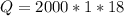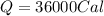Physics, 11.07.2019 16:00 ryrytkg5107
How many "big c" calories does it take to raise the temperature of 2.00 l of water from 22.0 c to 40.0 c?

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:30
Match the atomic particles with their characteristics. 1. made of two protons and two neutrons neutron 2. similar to an electron; positive charge neutrino 3. orbits nucleus; negative charge positron 4. bundle of energy; zero charge proton 5. found in nucleus; positive charge meson 6. very unstable; +, -, or zero charge gamma 7. found in nucleus; zero charge electron 8. negative particle; negative charge beta 9. atomic energy converted to mass alpha
Answers: 1

Physics, 22.06.2019 05:10
What is the electric force acting between two charges of -0.0045 c and -0.0025 c that are 0.0060 m apart? use fe=kq1q2/r^2 and k = 9.00 x 10^9 n*m^2/c^2 a. 1.7 x 10^7 n b. -1.7 x 10^7 n c. -2.8 x 10^9 n d. 2.8 x 10^9 n
Answers: 1

Physics, 22.06.2019 05:40
Unpolarized light of intensity i_0=750w/m^2 is incident upon two polarizers. after passing through both polarizers the intensity is i_2=280w/m^2. (a) what is the intensity of the light after it passes through the first polarizer in w/m^2? (b) write an equation for the angle between the polarizers in terms of the initial (i_0) and final (i_2) intensities. (c) find the angle between the polarizers in degrees.
Answers: 3

Physics, 22.06.2019 07:00
Oxygen and hydrogen gas are at the same temperature t.what is the ratio of kinetic energies of oxygen molecule and hydrogen molecule if oxygen is 16 times heavier than hydrogen.
Answers: 3
You know the right answer?
How many "big c" calories does it take to raise the temperature of 2.00 l of water from 22.0 c to 40...
Questions

Mathematics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

Advanced Placement (AP), 18.11.2020 20:50

Arts, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50



Health, 18.11.2020 20:50

Physics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50


Arts, 18.11.2020 20:50


Mathematics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50


Law, 18.11.2020 20:50


English, 18.11.2020 20:50