
Physics, 12.07.2019 09:00 Spiderblack212
What tripositive ion has the electron configuration [kr] 4d3 ? what neutral atom has the electron configuration [kr] 5s 2 4d2 ?

Answers: 1


Another question on Physics

Physics, 22.06.2019 22:00
Aspaceship starting from a resting position accelerates at a constant rate of 9.8 meters per second per second. how far will the spaceship travel if its final speed is 1 percent the speed of light (300,000,000 m/s)? a. 3.1 × 106 m b. 2.94 × 108 m c. 7.8 × 1010 m d. 4.6 × 1013 m
Answers: 1

Physics, 23.06.2019 00:20
You are the coordinator for a program that is going to take place at night in a rectangular amphitheater in the mountains. you will have no access to any electricity, but you must be able to illuminate the entire grounds. you know the intensity of the light from a lantern varies inversely as the square of the distance from the lantern. suppose the intensity is 90 when the distance is 5 m. a. write an equation to model the situation. b. solve for the constant of variation. c. write the equation to model the situation using the constant () of variation. d. you have been given lanterns with 40 light intensity. use your equation to solve for the distance from the lantern. e. you need to illuminate 225 km. how many meters do you need to light? f. how many lanterns will you need?
Answers: 3

Physics, 23.06.2019 00:20
An object with a mass of 1.5 kg changes its velocity from + 15 m/s to +22 m/s during a time interval of 3.5 seconds. what impulse was delivered to the objects?
Answers: 2

Physics, 23.06.2019 06:30
If a 2 ounce coin is dropped from the top of the washington monument and has a final velocity of 300 mph (miles per hour) when it hits the ground, how much kinetic energy will it have at that time?
Answers: 2
You know the right answer?
What tripositive ion has the electron configuration [kr] 4d3 ? what neutral atom has the electron c...
Questions








Mathematics, 05.11.2020 17:00

Chemistry, 05.11.2020 17:00


Mathematics, 05.11.2020 17:00

English, 05.11.2020 17:00






Mathematics, 05.11.2020 17:00


Mathematics, 05.11.2020 17:00

![[Kr]4d^{3}](/tpl/images/0080/3631/df596.png)
![[Kr]4d^{5}5s^{1}](/tpl/images/0080/3631/76de8.png) .
.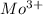 .
.![[Kr]5s^{2}4d^{2}](/tpl/images/0080/3631/10e60.png) .
.

