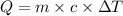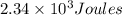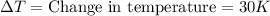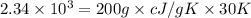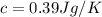Answers: 1


Another question on Physics

Physics, 22.06.2019 11:50
Amoving electron has kinetic energy k1. after a net amount of work w has been done on it, the electron is moving one-quarter as fast in the opposite direction. (a) find w in terms of k1. (b) does your answer depend on the final direction of the electron's motion?
Answers: 2

Physics, 22.06.2019 13:00
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.
Answers: 1

Physics, 22.06.2019 16:00
The process of predicting values that extend beyond the range of data points is called absolute value extrapolation interpolation prediction for any given: )
Answers: 2

Physics, 22.06.2019 17:00
Two conductors, a and b, are each in the shape of a tetrahedron. but of different sizes. they are charged in the following manner: 1. tetrahedron a is charged from an electrostatic generator to charge q. 2. tetrahedron a is briefly touched to tetrahedron b. 3. steps 1 and 2 are repeated until the charge on tetrahedron b reaches a maximum value. if the charge on tetrahedron b was q/4 after the first time it touched tetrahedron a. what is the final charge qbmax on tetrahedron b?
Answers: 2
You know the right answer?
A200.0 g copper block absorbs 2.34 × 10^3 j of heat to raise its temperature by 30.0 k. what is the...
Questions

History, 18.11.2019 03:31

Mathematics, 18.11.2019 03:31

Mathematics, 18.11.2019 03:31

Spanish, 18.11.2019 03:31



Mathematics, 18.11.2019 03:31


History, 18.11.2019 03:31

Spanish, 18.11.2019 03:31


Chemistry, 18.11.2019 03:31

Health, 18.11.2019 03:31


Mathematics, 18.11.2019 03:31


Mathematics, 18.11.2019 03:31

History, 18.11.2019 03:31

Mathematics, 18.11.2019 03:31