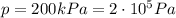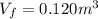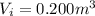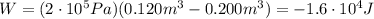Physics, 19.07.2019 02:30 lakenyahar
Acompression at a constant pressure of 200 kpa is performed on 8.00 moles of an ideal monatomic gas. the compression reduces the volume of the gas from 0.200 cubic meters to 0.120 cubic meters. develop a strategy to determine the amount of work that was done by the gas during this process?

Answers: 1


Another question on Physics

Physics, 21.06.2019 16:30
Aball bearing at 220.0 °c is dropped into a cup containing 250.0 g of water at 20.0 °c. the water and ball bearing come to a temperature of 30.0 °c. what is the heat capacity of the ball bearing in j/°c? (hint: how are the algebraic signs of q related for the ball bearing and the water? )
Answers: 1


Physics, 22.06.2019 15:00
Holes drilled several kilometers into earth’s crust provide direct evidence about earth’s interior in the form of
Answers: 1

Physics, 22.06.2019 15:50
Select all the correct answers. which changes will increase the rate of reaction during combustion? decreasing the area of contact between the reactants adding more oxygen to the reaction removing heat from the reaction changing the reactants from solid form to powdered form lowering the exposure of the reactants to air
Answers: 3
You know the right answer?
Acompression at a constant pressure of 200 kpa is performed on 8.00 moles of an ideal monatomic gas....
Questions

Mathematics, 07.12.2020 17:00

Mathematics, 07.12.2020 17:00

Social Studies, 07.12.2020 17:00


Social Studies, 07.12.2020 17:00




Mathematics, 07.12.2020 17:00

Chemistry, 07.12.2020 17:00





Mathematics, 07.12.2020 17:00



Computers and Technology, 07.12.2020 17:00


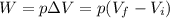
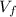 is the final volume of the gas
is the final volume of the gas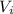 is the initial volume of the gas
is the initial volume of the gas