Mathematics, 11.05.2021 18:30 AquaTyrant9232
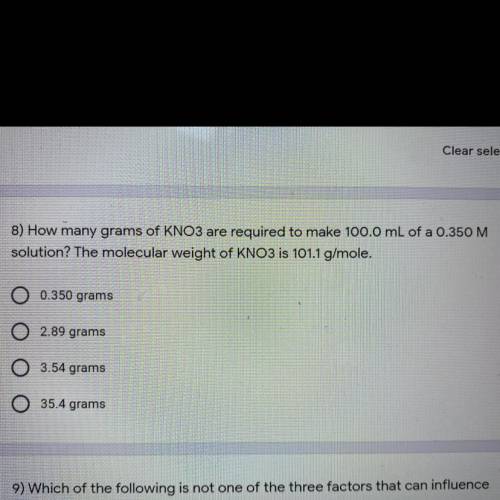
How many grams of KNO3 are required to make 100.0 mL of a 0.350 M solution? The molecular weight of KNO3 is 101.1 g/mole

Answers: 2


Another question on Mathematics


Mathematics, 21.06.2019 19:20
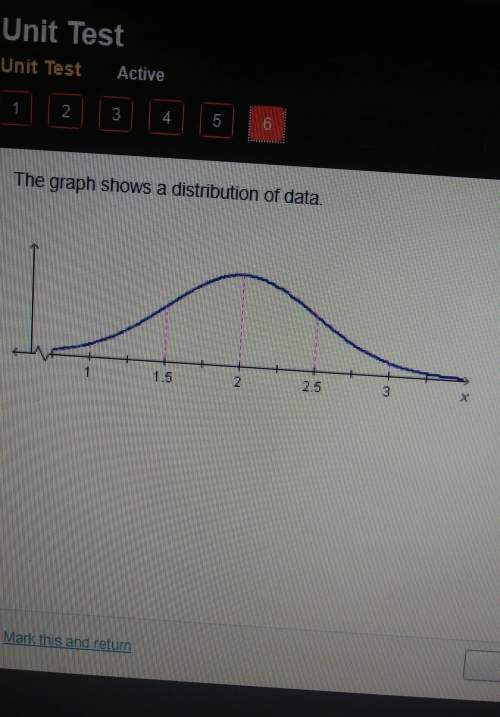
Which of the following is the result of expanding the series
Answers: 1

Mathematics, 21.06.2019 23:00
Find the equation of the ellipse with the following properties. the ellipse with foci at (0, 6) and (0, -6); y-intercepts (0, 8) and (0, -8).edit: the answer is x^2 over 28 + y^2 over 64 = 1
Answers: 2

Mathematics, 22.06.2019 00:20
What is the slope of the line passing through the points (3, 3) and (5, 7) ? 1. 2 2. 1/2 3. −2 4. −1/2
Answers: 2
You know the right answer?
How many grams of KNO3 are required to make 100.0 mL of a 0.350 M
solution? The molecular weight of...
Questions




Mathematics, 06.10.2019 19:30



History, 06.10.2019 19:30




Mathematics, 06.10.2019 19:30

Health, 06.10.2019 19:30



History, 06.10.2019 19:30



Mathematics, 06.10.2019 19:30


History, 06.10.2019 19:30