
Mathematics, 30.04.2021 19:50 Uhmjujiooo45701
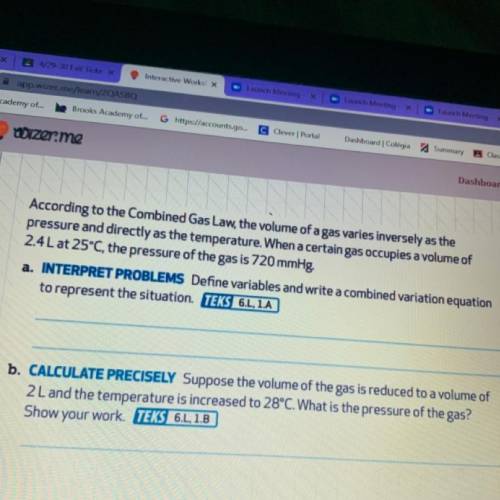
According to the Combined Gas Law, the volume of a gas varies inversely as the
pressure and directly as the temperature. When a certain gas occupies a volume of
2.4L at 25°C, the pressure of the gas is 720 mmHg?
a.
Define variables and write a combined variation equation
to represent the situation.
b. CALCULATE PRECISELY Suppose the volume of the gas is reduced to a volume of
2 L and the temperature is increased to 28°C. What is the pressure of the gas?
Show your work.

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 17:00
During your cycling trip, you and your friends often stayed in hostels. the prices of these are listed below. hostel standard room (price per night) with breakfast (price per night) super sleep hostels £22.45 £28.65 normandy nights £18.55 £25 zzzola hostels £24.50 £30.99 belgian beds £11.20 £18.55 night-time rest days ? £33 what is the range in cost for a single night, including both standard room prices and with breakfast prices?
Answers: 1

Mathematics, 21.06.2019 22:40
What rotation was applied to triangle def to create d’e’f’?
Answers: 2

Mathematics, 21.06.2019 23:30
Segment wx is shown explain how you would construct a perpendicular bisector of wx using a compass and a straightedge
Answers: 3

Mathematics, 22.06.2019 00:00
Mrs. blake's bill at a restaurant is $42.75. she wants to leave the waiter an 18% tip. how much will she pay in all, including the tip?
Answers: 2
You know the right answer?
According to the Combined Gas Law, the volume of a gas varies inversely as the
pressure and direct...
Questions

Social Studies, 29.01.2020 17:05

Geography, 29.01.2020 17:05

Mathematics, 29.01.2020 17:05


Geography, 29.01.2020 17:05

English, 29.01.2020 17:05


Mathematics, 29.01.2020 17:05




Social Studies, 29.01.2020 17:05


Physics, 29.01.2020 17:05


History, 29.01.2020 17:05






