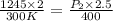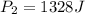Mathematics, 05.12.2019 10:31 laylah255
The pressure, p, of a gas varies directly with its temperature, t, and inversely with its volume, v, according to the equation p=nrt/v, where n is the number of molar units and r is the universal gas constant. one molar unit of gas has a pressure of about 1,245 joules at a temperature of 300 degrees kelvin and a volume of 2 liters. what is the pressure of the same number of molar units of the gas at a temperature of 400 degrees kelvin and a volume of 2.5 liters?

Answers: 3


Another question on Mathematics

Mathematics, 20.06.2019 18:04
Mr. vasquez determines that the area of a bathroom is in his house is 25 square feet less than one-fifth of the area of the living room if the bathroom measures 35 square feet what is the area of the living room
Answers: 1

Mathematics, 21.06.2019 17:40
If sec theta = 5/3 and the terminal point determined by theta is in quadrant 4, then
Answers: 1

Mathematics, 21.06.2019 18:00
How many glue sticks are in a basket containing 96 scissors, if the ratio of glue sticks to scissors is 19 to 57.
Answers: 1

Mathematics, 21.06.2019 19:20
Based on the diagram, match the trigonometric ratios with the corresponding ratios of the sides of the triangle. tiles : cosb sinb tanb sincposs matches: c/b b/a b/c c/a
Answers: 2
You know the right answer?
The pressure, p, of a gas varies directly with its temperature, t, and inversely with its volume, v,...
Questions

History, 25.09.2019 13:00

English, 25.09.2019 13:00

Health, 25.09.2019 13:00

Chemistry, 25.09.2019 13:00

English, 25.09.2019 13:00





Mathematics, 25.09.2019 13:00


History, 25.09.2019 13:00


Chemistry, 25.09.2019 13:00






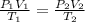 (for same value of n)
(for same value of n)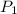 = initial pressure of gas = 1245 J
= initial pressure of gas = 1245 J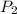 = final pressure of gas = ?
= final pressure of gas = ?
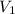 = initial volume of gas = 2 L
= initial volume of gas = 2 L
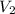 = final volume of gas = 2.5L
= final volume of gas = 2.5L
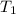 = initial temperature of gas = 300K
= initial temperature of gas = 300K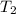 = final temperature of gas = 400K
= final temperature of gas = 400K