
Mathematics, 03.02.2021 03:50 mayaa2351
A mole of a chemical element contains approximately 6.02 x 10.23 atoms. Match the following moles of elements with the approximate number of atoms in them
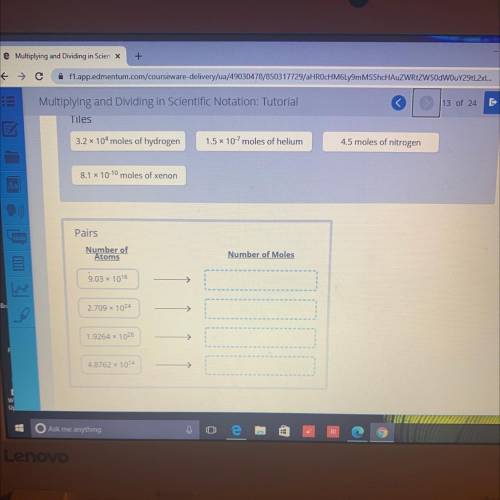

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 20:00
Evaluate the discriminant of each equation. tell how many solutions each equation has and whether the solutions are real or imaginary. x^2 - 4x - 5 = 0
Answers: 2

Mathematics, 21.06.2019 20:30
Pyramid shown has a square base that is 18 inches on each side has a surface area of 164 square inches what is the slant height
Answers: 3

Mathematics, 21.06.2019 21:00
Ariana starts with 100 milligrams of a radioactive substance. the amount of the substance decreases by 20% each week for a number of weeks, w. the expression 100(1−0.2)w finds the amount of radioactive substance remaining after w weeks. which statement about this expression is true? a) it is the difference between the initial amount and the percent decrease. b) it is the difference between the initial amount and the decay factor after w weeks. c) it is the initial amount raised to the decay factor after w weeks. d) it is the product of the initial amount and the decay factor after w weeks.
Answers: 1

Mathematics, 21.06.2019 22:00
Problem situation: caren is making rice and beans. she can spend no more than $10 on ingredients. she buys one bag of rice for $4.99. beans cost $0.74 per pound. how many pounds of beans, x, can she buy? inequality that represents this situation: 10≥4.99+0.74x drag each number to show if it is a solution to both the inequality and the problem situation, to the inequality only, or if it is not a solution.
Answers: 1
You know the right answer?
A mole of a chemical element contains approximately 6.02 x 10.23 atoms. Match the following moles of...
Questions



Mathematics, 27.03.2020 04:30






Mathematics, 27.03.2020 04:30



Mathematics, 27.03.2020 04:30



Mathematics, 27.03.2020 04:30

Chemistry, 27.03.2020 04:30

Computers and Technology, 27.03.2020 04:30





