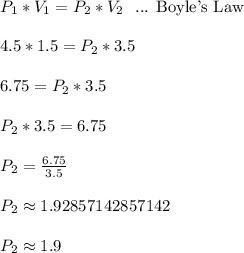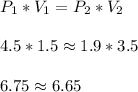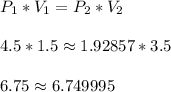Mathematics, 29.12.2020 20:30 ayoismeisalex
When a gas is kept at a constant temperature and pressure on it changes, its volume changes according to the following formula, known as Boyle’s law where P1 and V1 are the pressure (in atm) and the volume (in litres) at the beginning, and P2 and V2 are the pressure and the volume at the end. Find the final pressure P2 if V1 = 1.5 litres, P1 = 4.5 atm and V2 = 3.5 litres. Round to the nearest tenth of a atm.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 17:20
Which system of linear inequalities is represented by the graph? + l tv x-3y > 6 and y > 2x o x + 3y > 6 and y o x-3y > 6 and y> 2x o x + 3y > 6 and y > 2x + 4 la +
Answers: 1


Mathematics, 21.06.2019 22:00
Asquare and an equilateral triangle have the same perimeter. each side the triangle is 4 inches longer than each side of the square. what is the perimeter of the square
Answers: 1

Mathematics, 21.06.2019 23:30
The average daily maximum temperature for laura’s hometown can be modeled by the function f(x)=4.5sin(πx/6)+11.8 , where f(x) is the temperature in °c and x is the month. x = 0 corresponds to january.what is the average daily maximum temperature in may? round to the nearest tenth of a degree if needed.use 3.14 for π .
Answers: 1
You know the right answer?
When a gas is kept at a constant temperature and pressure on it changes, its volume changes accordin...
Questions

Mathematics, 28.10.2020 16:00

Mathematics, 28.10.2020 16:00


English, 28.10.2020 16:00


Mathematics, 28.10.2020 16:00

Mathematics, 28.10.2020 16:00

Biology, 28.10.2020 16:00

Mathematics, 28.10.2020 16:00





Mathematics, 28.10.2020 16:00






Law, 28.10.2020 16:00