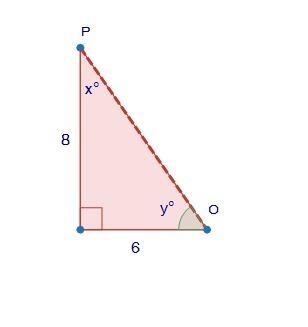
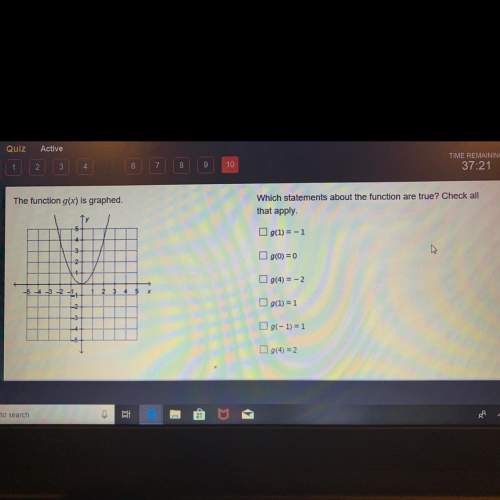
Mathematics, 18.11.2020 01:00 20dyeaubn
Find the amount of energy (Q) required to raise the temperature of the water in kilo joules (KJ). Given mass of water m = 2kg = 2000g. Temperature difference ΔΤ = T2 – T1 = 60 °C – 25 °C = 35 °C . Specific heat of water = 4.18 J/g* °C .

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 20:00
The radius of the earth is two times the radius of the moon. what fraction of the volume of the earth is the volume of the moon?
Answers: 1

Mathematics, 21.06.2019 20:20
Select the correct answer. what is the exact value of sin (157.5°)? a. 'sqrt(2 - sqrt(2))/2 b. *"-"'sqrt(2 + sqrt(2))/29 c.'sqrt(2 + sqrt(2))/4" d. "-"sqrt(2 + sqrt(2))/4)
Answers: 3


Mathematics, 22.06.2019 00:00
Tony is charged $ 50 and additional $0.15 per miles for renting a car. a) represent the cost of renting a car with an equation,and the determine the cost if he drove it70 miles.b) what would be the cost of a car rental if the car was driven 250 miles? show work
Answers: 1
You know the right answer?
Find the amount of energy (Q) required to raise the temperature of the water in kilo joules (KJ).
G...
Questions


Mathematics, 12.07.2019 20:30


Computers and Technology, 12.07.2019 20:30

English, 12.07.2019 20:30


Biology, 12.07.2019 20:30





Mathematics, 12.07.2019 20:30


Mathematics, 12.07.2019 20:30


Health, 12.07.2019 20:30


Mathematics, 12.07.2019 20:30


History, 12.07.2019 20:30