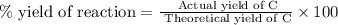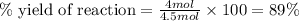Mathematics, 26.08.2019 10:50 purplefive85
Consider the following reaction: 2a + b -> 3c + d 3.0 mol a and 2.0 mol b react to form 4.0 mol c. what is the percent yield of this reaction?
a) 50%
b) 67%
c) 75%
d) 89%
e) 100%
what other formulas for % yield other than actual/theoretical yield?

Answers: 1


Another question on Mathematics


Mathematics, 21.06.2019 21:20
Drag each expression to the correct location on the solution. not all expressions will be used. consider the polynomial 8x + 2x2 - 20x - 5. factor by grouping to write the polynomial in factored form.
Answers: 1

Mathematics, 21.06.2019 23:00
Is there a direction u in which the rate of change of f(x,y)equals=x squared minus 3 xy plus 4 y squaredx2−3xy+4y2 at p(1,2) equals 14? give reasons for your answer. choose the correct answer below. a. no. the given rate of change is smaller than the minimum rate of change. b. no. the given rate of change is larger than the maximum rate of change. c. yes. the given rate of change is larger than the minimum rate of change and smaller than the maximum rate of change.
Answers: 2

Mathematics, 21.06.2019 23:00
Which of the following graphs could represent a cubic function?
Answers: 1
You know the right answer?
Consider the following reaction: 2a + b -> 3c + d 3.0 mol a and 2.0 mol b react to form 4.0 mol...
Questions



Physics, 12.08.2020 05:01



Mathematics, 12.08.2020 05:01






Mathematics, 12.08.2020 05:01





Computers and Technology, 12.08.2020 05:01



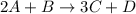
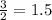 moles of B
moles of B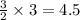 mole of C
mole of C