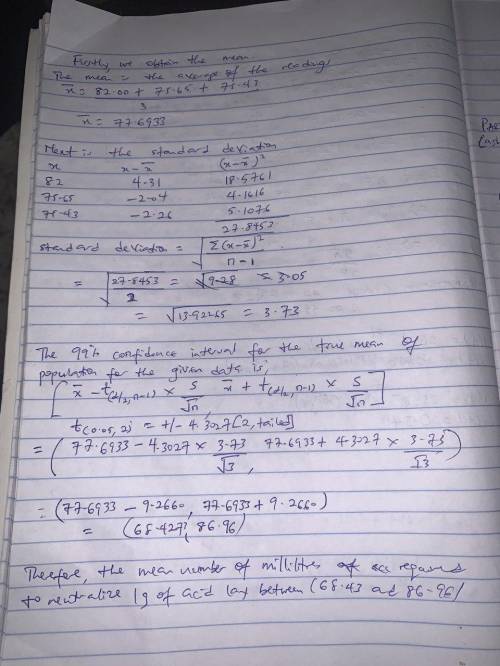Mathematics, 06.05.2020 07:09 jack1043
The object of a general chemistry experiment is to determine the amount (in milliliters) of sodium hydroxide (NaOH) solution needed to neutralize 1 gram of a specified acid. This will be an exact amount, but when the experiment is run in the laboratory, variation will occur as the result of experimental error. Three titrations are made using phenolphthalein as an indicator of the neutrality of the solution (pH equals 7 for a neutral solution). The three volumes of NaOH required to attain a pH of 7 in each of the three titrations are as follows: 82.00, 75.65, and 75.43 milliliters.
Required:
Use a 99% confidence interval to estimate the mean number of milliliters required to neutralize 1 gram of the acid

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 17:20
Aboat costs 19200 and decreases in value by 12% per year. how much will it cost in 5 years
Answers: 2

Mathematics, 21.06.2019 20:50
Samson is going shopping for sugar and butter to make three different kinds of cookies: lemon bars, peanut butter cookies, and sugar cookies. the recipe for lemon bars calls for 2 cups of flour, 2 cups of sugar, and 1 stick of butter. the peanut butter cookie recipe calls for 2 cups of flour, 4 cup of sugar and 2 stick of butter. the sugar cookie recipe calls for 1 cup of flour, 2 cups of sugar, and 2 sticks of butter. sampson has 13 cups of flour at home, and he buys 12 cups (6 pounds) of sugar and 10 sticks of butter. how many batches of each type of cookie can he make? samson can make batches of lemon bars, batches of peanut butter cookies, and batches of 09 cookies submit reset
Answers: 1

Mathematics, 21.06.2019 21:00
The perimeter of a rectangle is 42 inches. if the width of the rectangle is 6 inches, what is the length
Answers: 2

Mathematics, 22.06.2019 00:50
The students in a class were asked how many siblings they have. the data obtained is represented in the dot plot. the number of students who have no siblings is . the number of students who have three or more siblings is .
Answers: 1
You know the right answer?
The object of a general chemistry experiment is to determine the amount (in milliliters) of sodium h...
Questions





Physics, 26.02.2021 23:20


Mathematics, 26.02.2021 23:20

History, 26.02.2021 23:20

Chemistry, 26.02.2021 23:20



Mathematics, 26.02.2021 23:20

Mathematics, 26.02.2021 23:20

Mathematics, 26.02.2021 23:20

Mathematics, 26.02.2021 23:20

Mathematics, 26.02.2021 23:20

Mathematics, 26.02.2021 23:20

Mathematics, 26.02.2021 23:20