Mathematics, 24.04.2020 04:31 janessa0804
In a one electron system, the probability of finding the electron within a shell of thickness rδr at a radius of rr from the nucleus is given by the radial distribution function, P(r)=r2R2(r)P(r)=r2R2(r) . An electron in a 1s1s hydrogen orbital has the radial wavefunction R(r)R(r) given by R(r)=2(1a0)3/2e−r/a0 R(r)=2(1a0)3/2e−r/a0 where a0a0 is the Bohr radius (52.9 pm)(52.9 pm) .
Calculate the probability of finding the electron in a sphere of radius 1.1a01.1a0 centered at the nucleus.

Answers: 2


Another question on Mathematics


Mathematics, 21.06.2019 17:30
What is the shape of the height and weight distribution
Answers: 2


Mathematics, 21.06.2019 20:30
Tom is the deli manager at a grocery store. he needs to schedule employee to staff the deli department for no more that 260 person-hours per week. tom has one part-time employee who works 20 person-hours per week. each full-time employee works 40 person-hours per week. write and inequality to determine n, the number of full-time employees tom may schedule, so that his employees work on more than 260 person-hours per week. graph the solution set to this inequality.
Answers: 2
You know the right answer?
In a one electron system, the probability of finding the electron within a shell of thickness rδr at...
Questions

English, 16.01.2021 01:00


Advanced Placement (AP), 16.01.2021 01:00


History, 16.01.2021 01:00

Mathematics, 16.01.2021 01:00

Mathematics, 16.01.2021 01:00



Mathematics, 16.01.2021 01:00




Geography, 16.01.2021 01:00


Mathematics, 16.01.2021 01:00

Mathematics, 16.01.2021 01:00

Biology, 16.01.2021 01:00

Social Studies, 16.01.2021 01:00

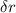 at a radius of r from the nucleus is given by the radial distribution function P (r) = r²R²(r)
at a radius of r from the nucleus is given by the radial distribution function P (r) = r²R²(r)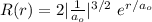 where
where 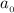 is the Bohr radius (52.9 pm)
is the Bohr radius (52.9 pm)![\\ \\ \\Probability \ (P) = \int\limits^b_0 \ P({r}) \, \ dr \\ \\ \\ where \ b= 1.1a__0}} \\ \\ = \int\limits^b_0 r^2 \ 4 (\frac{1}{a__0}})^3 \ e^{-2r/a__0}} \ dr \\ \\ \\ = \frac{4}{a^3__0}}} \ \int\limits^b_r r^2 \ e^{-2r/a__0}} \ dr \\ \\ \\ = \frac{4}{a^3__0}}} [(r^2 \frac{e^{-2r/a_0}}{(-2/a_0_})}]^b___0}}} \ - \ \int\limits^b_0 2r \frac{e^{-2r/a}}{-2/a_0} \ dr]](/tpl/images/0623/4976/bdc8e.png)
![= \frac{4}{a__0}^3}[(\frac{a_0b^2e^{-2b/a_0}}{(-2)})+a_0\int\limits^b_0 \ r \ e^{-2r/a} \ dr] -------- equation (1)](/tpl/images/0623/4976/6c06f.png)
![\int\limits^b_0 \ r \ e^{-2r/a} \ dr \\ \\ \\ = [r \ \frac{e^{-2r/a}}{(-2/a)}]^b__0}}- \int\limits^b_0 \frac{e^{-2r/a}}{(-2/a)}} \\ \\ \\ = \frac{ab \ e^{-2b/a}}{(-2)} + \frac{a}{2} \ \int\limits^b_a \ e^{-2r/a} \ dr](/tpl/images/0623/4976/801a3.png)
![= \frac{ab \ e^{-2b/a}}{(-2)} + \frac{a}{2} \frac {[e^{-2r/a}]^b_0}{(-2/a)}](/tpl/images/0623/4976/7193d.png)
![\int\limits^b_0 \ r \ e^{-2r/a} \ dr = \frac{ab \ e^{-2b/a}}{(-2)} - \frac{a^2}{4}[e^{-2b/a}-1]](/tpl/images/0623/4976/4f852.png)
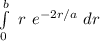 into equation (1); we have:
into equation (1); we have:![Probability (P) = \frac{4}{a__0}^3}[(\frac{a_0b^2e^{-2b/a_0}}{(-2)})+ \frac{ab \ e^{-2b/a}}{(-2)} - \frac{a^2}{4}[e^{-2b/a}-1]]](/tpl/images/0623/4976/4cb38.png)
![Probability (P) = \frac{4}{a^3}[(\frac{a^3(1.1)^2e^{-2(1.1)}}{(-2)})+ {a^2_3(1.1) \ e^{-2*1.1}} - \frac{a^3}{4}[e^{-2(1.1)}-1]]](/tpl/images/0623/4976/23b50.png)
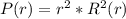
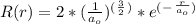
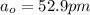 , is the Bohr's radius.
, is the Bohr's radius.![P ( r ) = \int [ 2*(\frac{1}{a_o} )^(^\frac{3}{2}^) * e^(^-^\frac{r}{a_o}^) ] ^2*r^2 dr\\\\P ( r ) = \int [ 4*(\frac{1}{a_o} )^(^3^) * e^(^-^\frac{2r}{a_o}^) ] *r^2 . dr\\\\P ( r ) = 4*(\frac{1}{a_o} )^(^3^) \int [ e^(^-^\frac{2r}{a_o}^) . r^2 ] . dr](/tpl/images/0623/4976/c5214.png)
![P ( r ) = 4*(\frac{1}{a_o} )^(^3^) * ( [ \frac{e^(^-^\frac{2r}{a_o}^) }{\frac{-2}{a_o} } ]*r^2 - \int [ e^(^-^\frac{2r}{a_o}^) . 2r ] . dr)](/tpl/images/0623/4976/d2121.png)