NH3(g) + Cl2(g) -> NH4Cl(s)

Mathematics, 18.04.2020 01:43 amison64
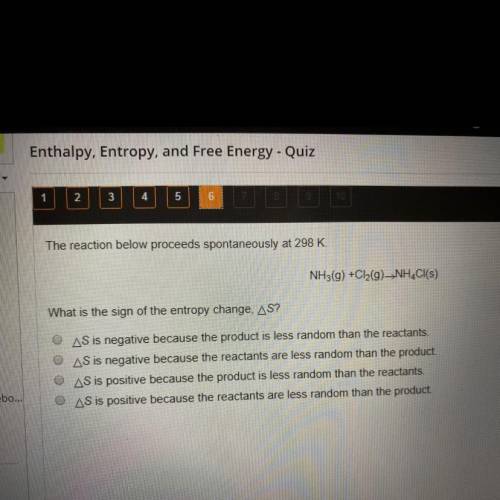
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
What is the sign of the entropy change, delta S?

Answers: 2


Another question on Mathematics


Mathematics, 21.06.2019 16:00
The scale for a map is 20 miles = 1/2 inch. the distance between two towns on the map is 3 3/4 inches. what is the actual distance between these towns? 150 miles 38 miles 75 miles 135 miles
Answers: 3

Mathematics, 21.06.2019 18:00
Write an equation for the function that includes the points (1,4/5) and (2,2/3)
Answers: 1

Mathematics, 21.06.2019 20:30
Arectangle has a width of 5 cm and a length of 10 cm. if the width is increased by 3, how does the perimeter change?
Answers: 1
You know the right answer?
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
NH3(g) + Cl2(g) -> NH4Cl(s)
Questions







History, 06.10.2019 18:30


Physics, 06.10.2019 18:30


Social Studies, 06.10.2019 18:30







World Languages, 06.10.2019 18:30

English, 06.10.2019 18:30

History, 06.10.2019 18:30




