2KCl(s) + 302) - 2KCIO3(s)
AH, KA - -435.9 kJ/mol
AHKCIO, - -391.4 kJ/mol
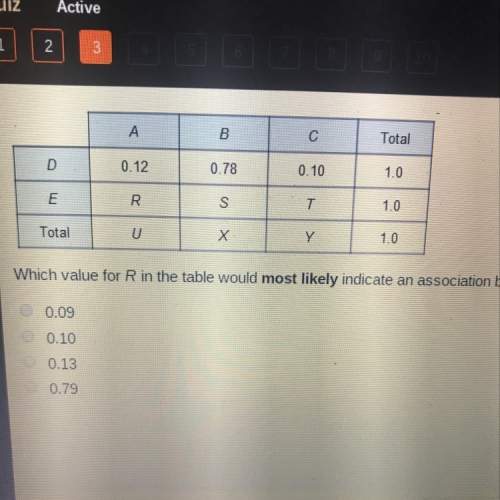
Which of...

Mathematics, 07.04.2020 07:06 Lalagrl
2KCl(s) + 302) - 2KCIO3(s)
AH, KA - -435.9 kJ/mol
AHKCIO, - -391.4 kJ/mol
Which of these are true for the reaction above? Check all that apply.
a. Hxn is 89.0kJ.
b. AHxn is-827.3kJ.
c. The reaction is endothermic, +AH.
d. The reaction is exothermic, -AH.
d. Heat is a reactant.

Answers: 2


Another question on Mathematics


Mathematics, 22.06.2019 00:30
What is the mean of the data set 125, 141, 213, 155, 281
Answers: 2

Mathematics, 22.06.2019 01:10
|z| > (1/2) {-1/2, 1/2} {z|(-1/2) < z < (1/2)} {z|z < (-1/2) ∪ z > (1/2)}
Answers: 3

Mathematics, 22.06.2019 01:30
Which shaded region is the solution to the system of inequalities? y y[tex]\geq[/tex]-x+1
Answers: 3
You know the right answer?
Questions

Mathematics, 03.09.2020 19:01


Mathematics, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01




Mathematics, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01




English, 03.09.2020 19:01



Mathematics, 03.09.2020 19:01


Computers and Technology, 03.09.2020 19:01

Physics, 03.09.2020 19:01

Mathematics, 03.09.2020 19:01