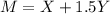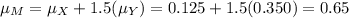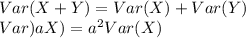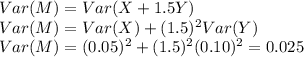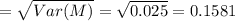Mathematics, 04.04.2020 10:54 Sbeech7362
The molarity of a solute in solution is defined to be the number of moles of solute per liter of solution (1 mole = 6.02 × 1023 molecules). If X is the molarity of a solution of magnesium chloride (MgCl2), and Y is the molarity of a solution of ferric chloride (FeCl3), the molarity of chloride ion (Cl−) in a solution made of equal parts of the solutions of MgCl2 and FeCl3 is given by M = X + 1.5Y . Assume that X has mean 0.125 and standard deviation 0.05, and that Y has mean 0.350 and standard deviation 0.10.
a. Find the mean of M
b. Find the standard deviation of M (assume X and Y are independent).
Please help, need accurate solutions

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 15:00
Asap the total attendance for all of a baseball league in 2012 was about 7.5×107 fans, while the attendance for the tornadoes in 2012 was about 1.5×106 fans. about how many times more was the attendance for the entire baseball league than the attendance for just the tornadoes? 50 times more 10 times more 2 times more 5 times more
Answers: 2

Mathematics, 21.06.2019 15:30
Franco wants to double the volume of the cone. what should he do?
Answers: 2

Mathematics, 21.06.2019 16:00
What is the value of x? enter your answer in the box. x = two intersecting tangents that form an angle of x degrees and an angle of 134 degrees.
Answers: 3

Mathematics, 21.06.2019 16:30
In two or more complete sentences, formulate how to use technology to calculate the appropriate regression model for the given data. you are not required to find the model, just choose the appropriate regression and explain how to use the technology. (-5,,2.,0.8), (0,-0.5), (2,-1.3), (3,-0.8), (5,2)
Answers: 2
You know the right answer?
The molarity of a solute in solution is defined to be the number of moles of solute per liter of sol...
Questions


Biology, 01.02.2021 22:30

Mathematics, 01.02.2021 22:30



Mathematics, 01.02.2021 22:30

English, 01.02.2021 22:30

Mathematics, 01.02.2021 22:30


Geography, 01.02.2021 22:30


Biology, 01.02.2021 22:30








Mathematics, 01.02.2021 22:40