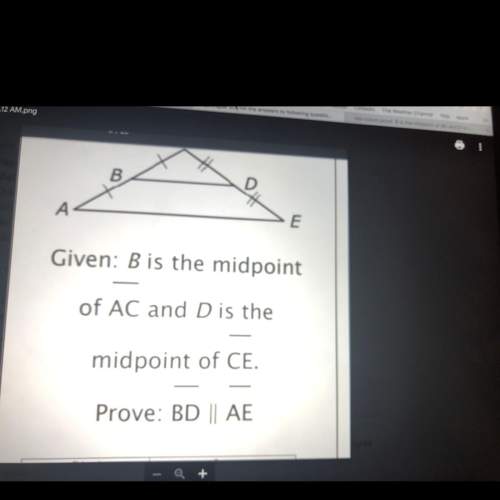
A chemist pumps hydrogen gas (H2) from a tank
into a reaction vessel containing 0.734 mol O2.<...

Mathematics, 01.04.2020 07:05 makayladurham19
A chemist pumps hydrogen gas (H2) from a tank
into a reaction vessel containing 0.734 mol O2.
The gases react to form water. If all of the available
O, reacts, how many moles of H2 are used?

Answers: 3


Another question on Mathematics


Mathematics, 21.06.2019 21:30
Look at triangle wxy what is the length (in centimeters) of the side wy of the triangle?
Answers: 1

Mathematics, 22.06.2019 00:30
Consider this expression and the steps to evaluate it. 4^5(−2)^9/4^8(−2)^3 1. apply the quotient of powers: (−2)^a/4^b 2. evaluate powers: c/d select the value of each variable. a = _ b = _ c = _ d = _
Answers: 3

Mathematics, 22.06.2019 02:00
Ven the functions, f(x) = 5x2 - 3x + 1 and g(x) = 2x2 + x - 2, perform the indicated operation. when applicable, state the domain restriction. (f - g)(x) 3x2 - 4x + 3 3x2 - 2x - 1 3x2 - 4x - 1 3x2 - 2x + 3
Answers: 3
You know the right answer?
Questions

English, 12.02.2021 20:20


Mathematics, 12.02.2021 20:20

Social Studies, 12.02.2021 20:20

Mathematics, 12.02.2021 20:20


Mathematics, 12.02.2021 20:20

Spanish, 12.02.2021 20:20

Mathematics, 12.02.2021 20:20

Mathematics, 12.02.2021 20:20


Mathematics, 12.02.2021 20:20

Mathematics, 12.02.2021 20:20


English, 12.02.2021 20:20



History, 12.02.2021 20:20

Mathematics, 12.02.2021 20:20