
Mathematics, 26.03.2020 16:36 mmaglaya1
The volume of an ideal gas is increased from 0.61 m3 to 3.9 m3 while maintaining a constant pressure of 970 Pa (1 Pa = 1 N/m2). If the initial temperature of the gas is 65 K, (a) What is the final temperature of the gas?

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 14:40
Write the sentence as an equation. z plus 372 is equal to 160
Answers: 2

Mathematics, 21.06.2019 19:00
Acompany manufactures large valves, packed in boxes. a shipment consists of 1500 valves packed in 75 boxes. each box has the outer dimensions 1.2 x 0.8 x 1.6 m and the inner dimensions 1.19 x 0.79 x 1.59 m. the boxes are loaded on a vehicle (truck + trailer). the available capacity in the vehicle combination is 140 m3. each valve has a volume of 0.06 m3. - calculate the load factor on the box level (%). - calculate the load factor on the vehicle level (%). - calculate the overall load factor (%).
Answers: 1

Mathematics, 22.06.2019 01:10
|y + 2| > 6 {y|y < -8 or y > 4} {y|y < -6 or y > 6} {y|y < -4 or y > 4}
Answers: 2

Mathematics, 22.06.2019 01:30
The population of a bacteria colony grows by a consistent percentage each hour and can be modeled by the function y = 500(1.16)t. what does the value 500 represent in the function?
Answers: 2
You know the right answer?
The volume of an ideal gas is increased from 0.61 m3 to 3.9 m3 while maintaining a constant pressure...
Questions

Mathematics, 26.04.2021 21:30

Mathematics, 26.04.2021 21:30

Mathematics, 26.04.2021 21:30

French, 26.04.2021 21:30


English, 26.04.2021 21:30


Mathematics, 26.04.2021 21:30


Advanced Placement (AP), 26.04.2021 21:30


Mathematics, 26.04.2021 21:30



Social Studies, 26.04.2021 21:30

Social Studies, 26.04.2021 21:30

Mathematics, 26.04.2021 21:30

History, 26.04.2021 21:30


Mathematics, 26.04.2021 21:30

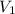 = 0.61 m^3 => 610L
= 0.61 m^3 => 610L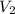 = 3.9 m^3 => 3900L
= 3.9 m^3 => 3900L 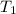 = 65 K
= 65 K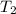 =?
=? 

