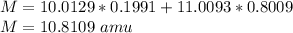Mathematics, 16.03.2020 23:47 oliviakate9230
Natural sample of boron consists of two isotopes. One has an exact mass of 10.0129 amu and its percent abundance is 19.91. The other isotope, of mass 11.0093 amu, has a percent abundance of 80.09. Calculate the average atomic mass.

Answers: 1


Another question on Mathematics


Mathematics, 21.06.2019 17:30
Me with this one question, and i'll upvote the brainliest answer
Answers: 2

Mathematics, 21.06.2019 21:00
Isabel graphed the following system of equations. 2x – y = 6 y = -3x + 4 she came up with the solution (2,-2). what were the 3 steps she did to get that solution? (make sure they are in the correct order)
Answers: 2

You know the right answer?
Natural sample of boron consists of two isotopes. One has an exact mass of 10.0129 amu and its perce...
Questions



History, 25.12.2019 23:31

English, 25.12.2019 23:31

History, 25.12.2019 23:31


Social Studies, 25.12.2019 23:31

Social Studies, 25.12.2019 23:31


Mathematics, 25.12.2019 23:31

Health, 25.12.2019 23:31

Biology, 25.12.2019 23:31

Computers and Technology, 25.12.2019 23:31



Mathematics, 25.12.2019 23:31


Mathematics, 25.12.2019 23:31

Mathematics, 25.12.2019 23:31