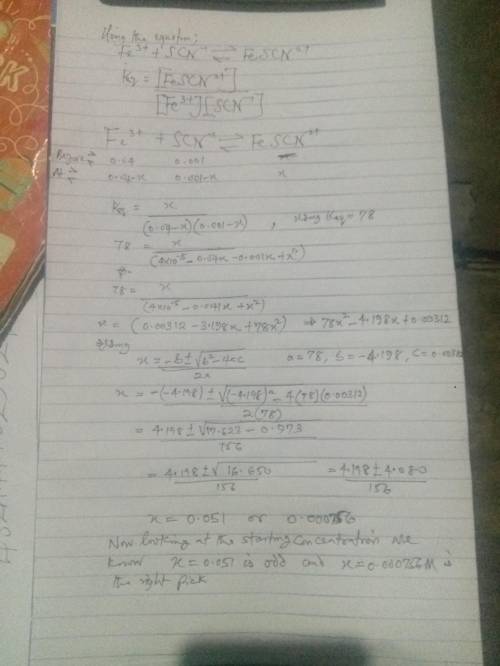Mathematics, 11.02.2020 21:27 Student2499
Using your calculated average value for Keq , calculate the SCN- concentration in a solution with an initial Fe3+ concentration of 4.00 x 10-2 M and the initial concentration of SCN- was 1.00 x 10-3 M. Is all the SCN- ion in the form of FeSCN2+? Hint: Set your product concentration to "x" and use the quadratic equation to solve. You will need to rearrange your Keq equation into the form of ax2 + bx + c = 0

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 17:00
Explain how you do each step what term makes it inconsistent y=2x - 4 ?
Answers: 1

Mathematics, 21.06.2019 19:00
Astore has clearance items that have been marked down by 60%. they are having a sale, advertising an additional 55% off clearance items. what percent of the original price do you end up paying?
Answers: 1

Mathematics, 21.06.2019 20:30
If there is 20 dogs in the shelter and 5 dogs get homes, and then 43 more dogs come. how many dogs are there in the shelter?
Answers: 1

You know the right answer?
Using your calculated average value for Keq , calculate the SCN- concentration in a solution with an...
Questions



Computers and Technology, 05.07.2021 19:30


History, 05.07.2021 19:30








History, 05.07.2021 19:30

Mathematics, 05.07.2021 19:30

History, 05.07.2021 19:30

Mathematics, 05.07.2021 19:30

English, 05.07.2021 19:30

Social Studies, 05.07.2021 19:30


Mathematics, 05.07.2021 19:30