
Mathematics, 16.01.2020 07:31 lexireyne2005
The element bromine exists in nature as two isotopes: 79br has a mass of 78.9183 u, and 81br has a mass of 80.9163 u. the average atomic mass of bromine is 79.90 u. calculate the relative abundance (as percentages) of the two bromine isotopes.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 13:00
The chart below shows the distribution of weeds in yard
Answers: 2

Mathematics, 21.06.2019 21:00
Atorch and a battery cost 2.50 altogether.the torch costs 1.50 more than the battery.what fraction of the total price is the torch? give your answer in its simplest form.
Answers: 2

Mathematics, 21.06.2019 21:30
Find the domain and range of the following function f(x) = 51x - 21+ 4
Answers: 2

Mathematics, 21.06.2019 21:30
Zack notices that segment nm and segment pq are congruent in the image below: which step could him determine if δnmo ≅δpqr by sas? (5 points) segment mo ≅ segment qr segment on ≅ segment qp ∠ n ≅ ∠ r ∠ o ≅ ∠ q
Answers: 3
You know the right answer?
The element bromine exists in nature as two isotopes: 79br has a mass of 78.9183 u, and 81br has a...
Questions

Mathematics, 05.06.2020 21:02





Physics, 05.06.2020 21:02



Mathematics, 05.06.2020 21:02


Computers and Technology, 05.06.2020 21:02



History, 05.06.2020 21:02


Mathematics, 05.06.2020 21:02




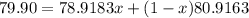
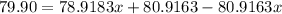
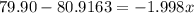
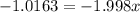
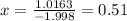
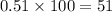 %
%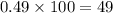 %
%


