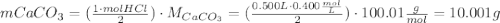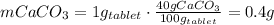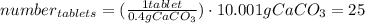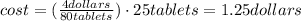Mathematics, 23.11.2019 02:31 davisdarby2
One container of tums® costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tums® is 40.0 percent caco₃ by mass. using only tums®, you are required to neutralize 0.500 l of 0.400 m hcl. how much does this cost? assume you are able to purchase individual tablets. express your answer in dollars.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 16:30
Answer the following for 896.31 cm= km 100cm = 1m 1000m = 1km a) 0.0089631 b) 0.0089631 c) 8.9631 d) 89.631
Answers: 1

Mathematics, 21.06.2019 19:00
You earn a 12% commission for every car you sell. how much is your commission if you sell a $23,000 car?
Answers: 1

Mathematics, 21.06.2019 22:00
The sum of the speeds of two trains is 720.2 mph. if the speed of the first train is 7.8 mph faster than the second train, find the speeds of each.
Answers: 1

Mathematics, 22.06.2019 00:30
What is the value of the discrimination for the quadratic equation 0=×2+2+×2
Answers: 2
You know the right answer?
One container of tums® costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tum...
Questions

History, 31.12.2019 01:31

Mathematics, 31.12.2019 01:31


Chemistry, 31.12.2019 01:31


Mathematics, 31.12.2019 01:31

Mathematics, 31.12.2019 01:31




Biology, 31.12.2019 01:31



Physics, 31.12.2019 01:31


Mathematics, 31.12.2019 01:31


History, 31.12.2019 01:31

Business, 31.12.2019 01:31

History, 31.12.2019 01:31