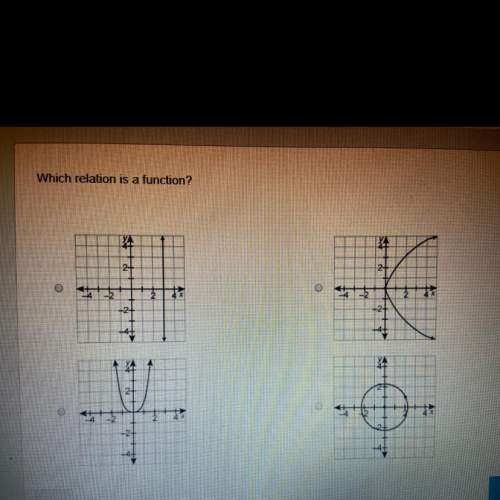
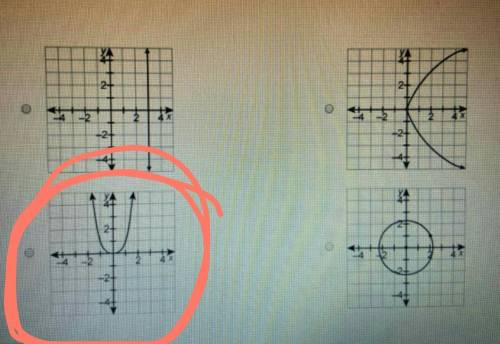
Which relation is a function?
...

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 23:00
Edger needs 6 cookies and 2 brownies for every 4 plates how many cookies and brownies does he need for 10 plates
Answers: 1

Mathematics, 22.06.2019 03:00
Describe how the presence of possible outliers might be identified on the following. (a) histograms gap between the first bar and the rest of bars or between the last bar and the rest of bars large group of bars to the left or right of a gap higher center bar than surrounding bars gap around the center of the histogram higher far left or right bar than surrounding bars (b) dotplots large gap around the center of the data large gap between data on the far left side or the far right side and the rest of the data large groups of data to the left or right of a gap large group of data in the center of the dotplot large group of data on the left or right of the dotplot (c) stem-and-leaf displays several empty stems in the center of the stem-and-leaf display large group of data in stems on one of the far sides of the stem-and-leaf display large group of data near a gap several empty stems after stem including the lowest values or before stem including the highest values large group of data in the center stems (d) box-and-whisker plots data within the fences placed at q1 â’ 1.5(iqr) and at q3 + 1.5(iqr) data beyond the fences placed at q1 â’ 2(iqr) and at q3 + 2(iqr) data within the fences placed at q1 â’ 2(iqr) and at q3 + 2(iqr) data beyond the fences placed at q1 â’ 1.5(iqr) and at q3 + 1.5(iqr) data beyond the fences placed at q1 â’ 1(iqr) and at q3 + 1(iqr)
Answers: 1

Mathematics, 22.06.2019 03:10
Which of the following statements are true? (select all that apply.) a quasi-static process is one in which the system is never far from being in equilibrium. when a system can go from state 1 to state 2 by several different processes, the amount of heat absorbed by the system will be the same for all processes. the internal energy of a given amount of an ideal gas depends only on its absolute temperature. when a system can go from state 1 to state 2 by several different processes, the work done on the system will be the same for all processes. when a system can go from state 1 to state 2 by several different processes, the change in the internal energy of the system will be the same for all processes. for any substance that expands when heated, its cp is greater than its cv.
Answers: 2

Mathematics, 22.06.2019 04:30
6points possible: 3. total attempts: 5 for the data shown, answer the questions. round to 2 decimal places. x 7.3 11.7 21.7 18.8 23.2 20.7 29.7 21.2 10.6 find the mean: find the median: find the standard deviation:
Answers: 2
You know the right answer?
Questions

Mathematics, 28.10.2020 20:10

Mathematics, 28.10.2020 20:10

History, 28.10.2020 20:10

Chemistry, 28.10.2020 20:10


Biology, 28.10.2020 20:10

English, 28.10.2020 20:10




Spanish, 28.10.2020 20:10

Health, 28.10.2020 20:10

Mathematics, 28.10.2020 20:10


Mathematics, 28.10.2020 20:10

Mathematics, 28.10.2020 20:10

Mathematics, 28.10.2020 20:10

History, 28.10.2020 20:10