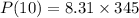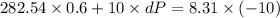Mathematics, 04.10.2019 21:20 tugordochulo1
The pressure, volume, and temperature of a mole of an ideal gas are related by the equation pv = 8.31t, where p is measured in kilopascals, v in liters, and t in kelvins. use differentials to find the approximate change in the pressure if the volume increases from 10 l to 10.6 l and the temperature decreases from 345 k to 335 k. (note whether the change is positive or negative in your answer. round your answer to two decimal places.)

Answers: 2


Another question on Mathematics

Mathematics, 20.06.2019 18:02
You paid $7.70 to the mail package that weighed 5.5 lb. right and solve any quetion to find the cost per pound ? ?
Answers: 1

Mathematics, 21.06.2019 19:00
Atriangle has a side lengths of 18cm, 80 cm and 81cm. classify it as acute obtuse or right?
Answers: 2

Mathematics, 21.06.2019 19:30
The revenue each season from tickets at the theme park is represented by t(c)=5x. the cost to pay the employees each season is represented by r(x)=(1.5)^x. examine the graph of the combined function for total profit and estimate the profit after four seasons
Answers: 3

Mathematics, 21.06.2019 20:00
The radius of the earth is two times the radius of the moon. what fraction of the volume of the earth is the volume of the moon?
Answers: 1
You know the right answer?
The pressure, volume, and temperature of a mole of an ideal gas are related by the equation pv = 8.3...
Questions




Chemistry, 07.10.2019 21:00

History, 07.10.2019 21:00

English, 07.10.2019 21:00


History, 07.10.2019 21:00

Mathematics, 07.10.2019 21:00

English, 07.10.2019 21:00

Mathematics, 07.10.2019 21:00

English, 07.10.2019 21:00





Physics, 07.10.2019 21:00