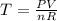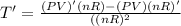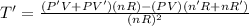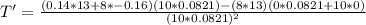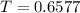Mathematics, 06.10.2019 01:00 Jsanders2276
The gas law for an ideal gas at absolute temperature t (in kelvins), pressure p (in atmospheres), and volume v (in liters) is pv = nrt, where n is the number of moles of the gas and r = 0.0821 is the gas constant. suppose that, at a certain instant, p = 8.0 atm and is increasing at a rate of 0.14 atm/min and v = 13 l and is decreasing at a rate of 0.16 l/min. find the rate of change of t with respect to time at that instant if n = 10 mol. (round your answer to four decimal places.)
 = k/min
= k/min

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 15:50
Dylan and dusty plan to take weekly surfing lessons together. if the 2-hour lessons are $20 per person and they plan to spend $100 each on new surfboards, what is the maximum number of lessons the two can take if the total amount spent for lessons and surfboards is at most $480?
Answers: 1


Mathematics, 22.06.2019 00:30
Given sin28.4=.4756, cos28.4=.8796, and tan28.4=.5407 find the cot of 61.6
Answers: 1

Mathematics, 22.06.2019 00:40
Find the volume of the solid bounded by the plane z=0 and the paraboloid z=1-x^2 –y^2
Answers: 1
You know the right answer?
The gas law for an ideal gas at absolute temperature t (in kelvins), pressure p (in atmospheres), an...
Questions


Mathematics, 24.09.2019 05:30

Mathematics, 24.09.2019 05:30




Mathematics, 24.09.2019 05:30






Spanish, 24.09.2019 05:30






History, 24.09.2019 05:30