
Mathematics, 01.08.2019 05:10 kactus
The standard cell potential (e°) of a voltaic cell constructed using the cell reaction below is 0.76 v: zn (s) + 2h+ (aq) → zn2+ (aq) + h2 (g) with ph2 = 1.0 atm and [zn2+] = 1.0 m, the cell potential is 0.52 v. the concentration of h+ in the cathode compartment is m.

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 19:30
Julian wrote the following numeric pattern on the board3,10,17,24,31,38.what numbers of julian's pattern are compound numbers.
Answers: 2

Mathematics, 21.06.2019 22:30
What is the graph of the absolute value equation ? y=|x|-5
Answers: 1

Mathematics, 22.06.2019 00:10
Answer asap and if you do you will get brainliest. catherine buys a gallon of ice cream from the store. after taking it home, she eats a fifth of a gallon of ice cream. her sister eats some of the ice cream as well. if two-thirds of the original amount of ice cream is left, then what fraction of a gallon of ice cream did her sister eat?
Answers: 2

Mathematics, 22.06.2019 00:30
How can you find the magnitude of a vector, v = < x,y > , where the horizontal change is x and the vertical change is y?
Answers: 1
You know the right answer?
The standard cell potential (e°) of a voltaic cell constructed using the cell reaction below is 0.76...
Questions

Advanced Placement (AP), 02.12.2021 06:40

History, 02.12.2021 06:40






Mathematics, 02.12.2021 06:40

English, 02.12.2021 06:40



English, 02.12.2021 06:40

English, 02.12.2021 06:40






Mathematics, 02.12.2021 06:40

Mathematics, 02.12.2021 06:40

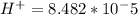 M
M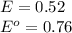
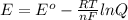
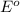 is the standard cell potential
is the standard cell potential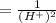
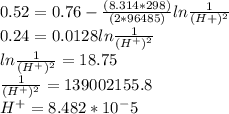 M
M

