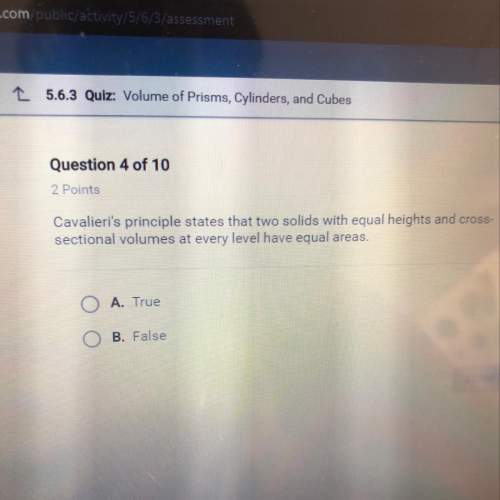
Mathematics, 23.06.2019 23:00 juliannasl
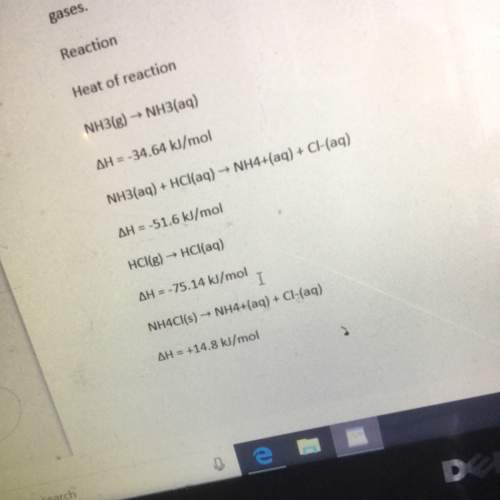
The heat of reaction for the process described in (a) can be determined by applying hess’s law. the heats of reaction shown in the table below can be obtained experimentally or looked up in tables of enthalpy data which two of these heats of reaction would be the easiest and safest to measure in the laboratory and which two are better obtained through reference sources why ? hint: consider whether a reaction takes place in aqueous solution or instead involves noxious gases. table is below

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 14:30
If anyone has done the algebra 2 chaos theory portfolio would you be wiling to me? i kind of know what i'm doing i just don't know if i'm doing it right.
Answers: 1

Mathematics, 21.06.2019 15:10
An objects motion is described by the equation d= 4sin (pi t) what will the height of the object be at 1.75 seconds?
Answers: 1

Mathematics, 21.06.2019 19:00
How can you tell when x and y are not directly proportional?
Answers: 1

Mathematics, 21.06.2019 23:30
If the perimeter of the garden is 37 ft. the width is x and the length is 15 ft. what is the width of the garden in feet?
Answers: 2
You know the right answer?
The heat of reaction for the process described in (a) can be determined by applying hess’s law. the...
Questions


History, 25.11.2019 01:31


Social Studies, 25.11.2019 01:31




Computers and Technology, 25.11.2019 01:31

Biology, 25.11.2019 01:31



Biology, 25.11.2019 01:31

History, 25.11.2019 01:31


Mathematics, 25.11.2019 01:31

Chemistry, 25.11.2019 01:31


English, 25.11.2019 01:31