
History, 02.05.2021 03:00 19thomasar
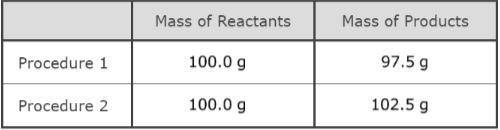
As part of an investigation, students combined substances in a beaker to observe chemical reactions. They performed two procedures. They measured the mass of each substance before and after each reaction. The table shows their observations.
Assuming the students did not make any careless errors, what likely explains these changes in mass?
A. Procedure 1: All the reactants were liquids that evaporated.
Procedure 2: A gas was formed as one product, and it escaped into the air.
B. Procedure 1: One of the reactants was converted to thermal energy.
Procedure 2: All the products were liquids.
C. Procedure 1: The reactants were liquids with different densities.
Procedure 2: The reactants were combined into only one product.
D. Procedure 1: One of the products was a gas that escaped into the air.
Procedure 2: A gas from the air reacted with one of the other reactants.
PLEASE ANSWER WITH EXPLINATION

Answers: 2


Another question on History

History, 21.06.2019 17:00
According to thomas hobbes concept of the social contract, what do people exchange for the protection by the government?
Answers: 1

History, 22.06.2019 04:00
Where did industrial capitalism originate and what advantages did this location have
Answers: 1

History, 22.06.2019 04:00
Enter the word you receive when you completed the river valley civilizations activity
Answers: 2

History, 22.06.2019 09:30
Which country joined the allies because it was a rival of germany?
Answers: 2
You know the right answer?
As part of an investigation, students combined substances in a beaker to observe chemical reactions....
Questions

Spanish, 22.04.2021 05:10



Mathematics, 22.04.2021 05:10

Arts, 22.04.2021 05:10

Mathematics, 22.04.2021 05:10

Mathematics, 22.04.2021 05:10





Chemistry, 22.04.2021 05:10

History, 22.04.2021 05:10


English, 22.04.2021 05:10

Mathematics, 22.04.2021 05:10

Arts, 22.04.2021 05:10


Mathematics, 22.04.2021 05:10



